Portal hypertension increase anti-donor immune response after living donor liver transplantation in patients and mouse model
Takashi Onoe1,2, Shinji Hashimoto2, Masataka Banshodani2, Kazuhiro Taguchi1,2, Yuka Tanaka2, Hideki Ohdan2.
1Institute for Clinical Research, National Hospital Organization, Kure Medical Center, Kure City, Japan; 2Department of Gastroenterological and Transplant Surgery, Hiroshima University, Hiroshima City, Japan
Background: Controlling portal vein pressure (PVP) in living donor liver transplantation (LDLT) has received increased attention owing to its potential importance for graft survival. However, immunological impact of PVP has not been elucidated. In this study, we investigated the impact of postoperative PVP on allo-immune response after LDLT and underlying mechanism using clinical data and mouse model, especially focusing on LSECs, which have immunological tolerogenic capacity.
Methods: We categorized 136 consecutive adult patients undergoing LDLT (excluding ABO-incompatible LDLT) to low- (<15 mmHg) and high-PVP (≥15 mmHg) groups, based on PVP values at the end of surgery and were retrospectively analyzed using the propensity score-matching method to compare anti-donor reaction and graft survival. To investigate the mechanism, we used 70% hepatectomized (HTx) mouse model with or without a porto-systemic shunt (PSS) to reproduce the clinical situation of portal hypertension in LDLT and we performed an allogeneic mixed hepatic constituent cell-lymphocyte reaction (MHLR). In this assay, hepatic single cell fraction from HTx Balb mice was used as stimulator. CFSE-dyed splenocytes from B6 mice was co-culture and anti-Balb response was quantified by their proliferation. In some experiment, phenotypic analysis and suppression assay using isolated LSECs were performed.
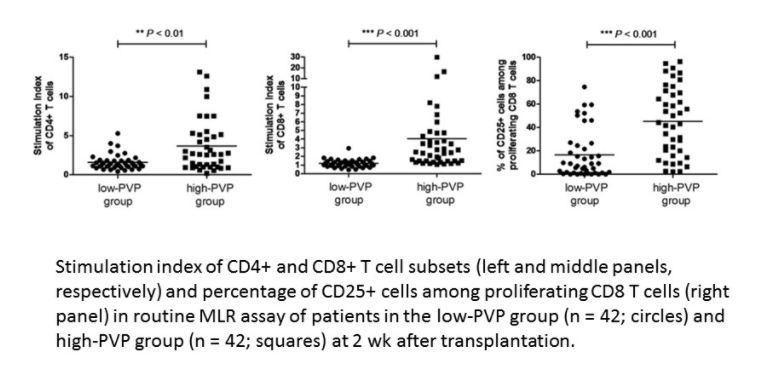
Results: In clinical study, 42 patient-pairs were selected for comparison. Routine mixed-lymphocyte reaction assays revealed that the high PVP group had a significantly higher anti-donor response of T cells than the low PVP group, at 2 weeks after LDLT (p < 0.01). Clinical rejection incidence of high-PVP group was significantly higher than low-PVP group within 3 months after LDLT (28.6% vs 9.5%, p < 0.05). Further, 2-year graft survival of high-PVP group was significantly lower than low-PVP group (92.9% vs 73.8%, p < 0.05). These clinical results suggests that portal hypertension enhanced anti-donor reaction and negatively affects graft survival after LDLT.
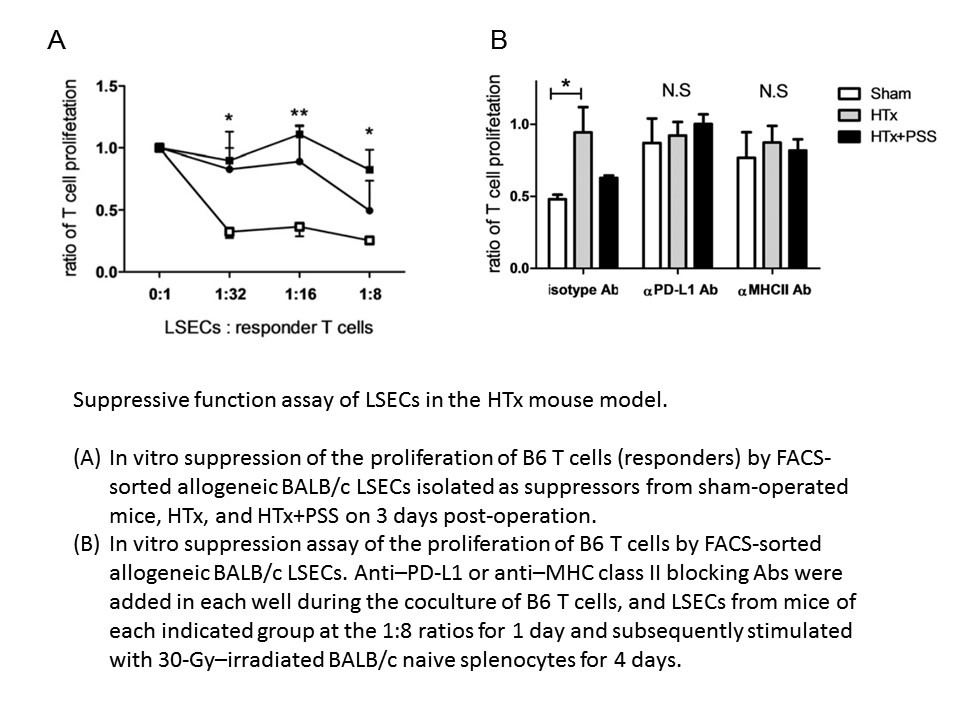
In mouse model, similar to clinical data, MHLR revealed that B6 T cells showed significantly higher proliferation in response to allogeneic hepatic single cells from HTx mice compared to that from sham-operated mice. Furthermore, PSS significantly suppressed this observed enhancement of T cell response (p < 0.001). Notably, LSECs from HTx mice exhibited significantly down-regulated MHC class II and PD-L1 expression. Subsequent suppression assay revealed that suppressive function was shown in LSECs from sham-operated mice while that was significantly decreased in LSECs from HTx mice. Blocking of MHC class II or PD-L1 by specific Abs abrogated the suppressive capacity of LSECs from sham-operated mice.
Conclusion: Results of this study suggest that postoperative portal hypertension enhances allo-immune responses in recipients after LDLT, likely due, in part, to the impaired immune-suppression capacity of LSECs.
There are no comments yet...