Efficacy and safety of everolimus with reduced calcineurin inhibitor regimen versus mycophenolic acid with standard calcineurin regimen in Asian de novo kidney transplant recipients: Two-year results from the subgroup analysis of TRANSFORM study
Yoshihiko Watarai1, Angel Joaquin Amante2, Concesa Casasola2, Myoung Soo Kim3, Shen-Shin Chang4, Duck Jong Han5, Atiporn Ingsathit6, Terence Kee7, Takashi Kenmochi8, Dinesh Khullar9, Prajej Ruangkanchanasetr10, Kuo-Hsiung Shu11, Hin Seng Wong12, Apurva Gawai13, Maria Pilar Hernandez-Gutierrez14, Romina Danguilan2.
1Department of Transplant Surgery, Kidney Disease Center, Japanese Red Cross Nagoya Daini Hospital , Nagoya-city, Japan; 2Department of Adult Nephrology, National Kidney and Transplant Institute, Quezon City, Philippines; 3Department of Surgery, Yonsei University College of Medicine and Severance Hospital, Seoul, Korea; 4Department of Surgery, National Cheng Kung University College of Medicine and Hospital, Tainan, Taiwan; 5Division of Kidney and Pancreas Transplantation, Department of Surgery, Asan Medical Center, Seoul, Korea; 6Faculty of Medicine , Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 7Department of Renal Medicine, Singapore General Hospital, Singapore, Singapore; 8Department of Organ Transplant Surgery, Fujita Health University Hospital, Toyoake-city, Japan; 9Department of Nephrology and Renal Transplant Medicine, Max Super Speciality Hospital, Saket , New Delhi, India; 10Division of Nephrology, Department of Medicine, Phramongkutklao Hospital, Bangkok, Thailand; 11Division of Nephrology, Taichung Veterans General Hospital, Taichung, Taiwan; 12Department of Nephrology and Clinical Research Centre , Clinical Research Centre Hospital Selayang, Selangor Darul Ehsan, Malaysia; 13Novartis India Ltd, Mumbai, India; 14Novartis Pharma AG, Basel, Switzerland
TRANSFORM Study group:Asian race sub-group analysis | Clinical trial identifier: NCT01950819.
Background: The TRANSFORM study has shown comparable efficacy-safety of everolimus with reduced calcineurin inhibitor (EVR+rCNI) versus mycophenolic acid and standard CNI (MPA+sCNI) treatment in de novo kidney transplant recipients (KTxRs). Here we present 24-month (M) data of the Asian race subgroup from the TRANSFORM study.
Methods: In this open-label study, KTxRs were randomized (1:1) to receive EVR+rCNI or MPA+sCNI; plus induction therapy and steroids. The primary composite endpoint was estimated glomerular filtration rate [Modification of Diet in Renal Disease] (eGFR [MDRD])<50 mL/min/1.73 m2 or treated biopsy-proven acute rejection (tBPAR). Other endpoints were composite efficacy failure (CEF: tBPAR, graft loss or death), evolution of eGFR, and safety. A multivariate analysis and a univariate analysis were performed retrospectively to further evaluate the factors affecting clinical outcomes.
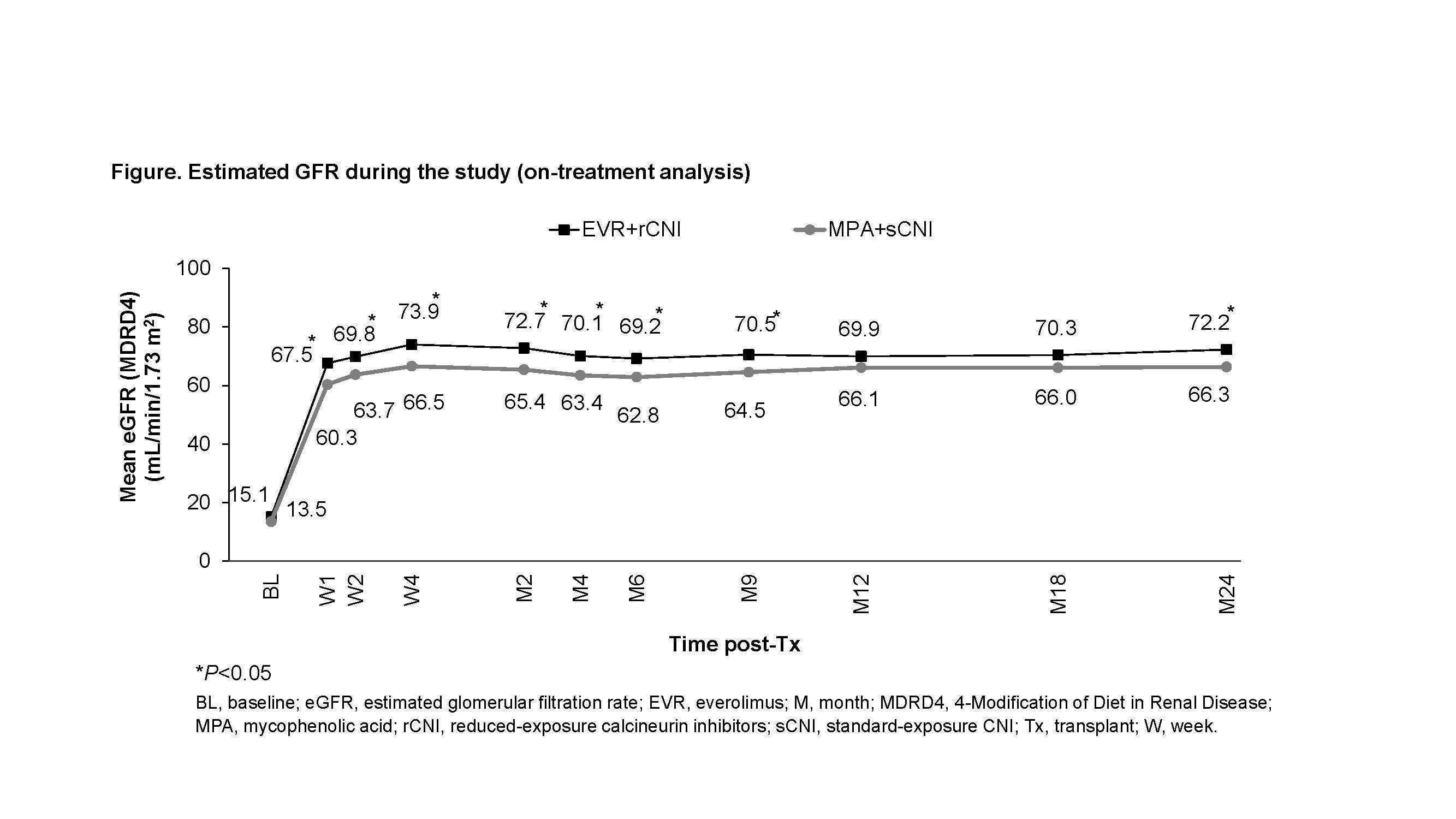
Results: Of 2037 randomized patients, 293 were of Asian race (EVR+rCNI: 136; MPA+sCNI: 157). The trough concentration (C0) for tacrolimus (up to 41.8% vs 27.8%) and cyclosporine (up to 63.2% vs 22.7%) was mostly above the target level in EVR+rCNI arm. At M24, EVR+rCNI arm was non-inferior to MPA+sCNI for the composite of eGFR (MDRD)<50 mL/min/1.73 m2 or tBPAR (27.0% vs 29.2%, P=0.011 for non-inferiority [NI] margin of 10%). Incidence of CEF was non-inferior in EVR+rCNI versus MPA+sCNI arm (9.0% vs 16.9%, P<0.001 for NI margin of 10%) and was driven by tBPAR (EVR+rCNI: 8.2% vs MPA+sCNI: 15.7%, P=0.068). Graft loss and death were reported for one patient each in both the treatment arms. Mean eGFR was significantly higher inEVR+rCNI versus MPA+sCNI (72.2 vs 66.3 mL/min/1.73 m2, P=0.041) (Figure). Multivariate analysis showed increasing donor age in EVR+rCNI (P=0.002) and MPA+sCNI arms (P<0.001), while deceased donor transplant (standard criteria donor, P=0.020) and use of cyclosporine (P=0.038) in MPA+sCNI arm as independent factors significantly impacting renal function. BMI of KTxRs had no effect on the clinical outcomes except for wound healing event (BMI<30 kg/m2: EVR+rCNI=26.3% vs MPA+sCNI=16.0%; P=0.040). Overall incidence of adverse events was comparable, with significantly lower incidence of BK virus (4.4% vs 12.1%) and cytomegalovirus infection (4.4% vs 13.4%) reported in EVR+rCNI arm.
Discussion: Better efficacy (CEF: 9.0% vs 18.0%; tBPAR: 8.2% vs 12.8%, respectively) and renal function (72.2 vs 58.2 mL/min/1.73 m2) observed in EVR+rCNI arm of the Asian subgroup versus overall population may be attributed to the higher proportion of living donors (84.6% vs 50.0%), younger recipients (43.3 vs 48.8 years) and donors (42.7 vs 48.3 years).
Conclusions: The M24 results from the subgroup analysis of the TRANSFORM study demonstrate that both treatment regimens in Asian KTxRs provide comparable antirejection efficacy with significantly better renal function and reduced viral infections in EVR+rCNI arm, with no new or unexpected safety concerns.
Novartis Pharma AG, Basel, Switzerland, supported this research.
There are no comments yet...