Effects of immunosuppression and adjuvant immune-targeted drugs in preventing HCC recurrence after liver transplantation
Hao Xing1, Jianhua Li1.
1Liver Transplant Centre, Department of General Surgery, Huashan Hospital, Fudan University, Shanghai, People's Republic of China
Liver Transplant Centre, Department of General Surgery, Huashan Hospital, Fudan University.
Introduction: Hepatocellular carcinoma (HCC) is an increasingly common indication for liver transplantation (LT), while post-transplant HCC recurrence is the main problem affecting progonosis of recipients. From 2014 to 2019, HCC was the indication for 45% of liver transplant registrants in our center, as the most common reason for LT. Post-transplantation preventive measures including the immunosuppression regimen like sirolimus, and immunotherapies like sorafenib and lenvatinib. In this study, we aimed to explore the effects of sirolimus(SRL)/tacrolimus(TAC) and sorafenib/lenvatinib in preventing recurrence.
Materials and Methods: This retrospective study including 207 recipients of liver transplants for hepatocellular carcinoma in Huashan hospitals affiliated to Fudan university. All recipients were followed up until December 2019 or until recurrence. The inclusion criteria: 1) histologically proven to be HCC; 2) without major vascular invasion. The exclusion criteria: 1) concurrent malignancy; 2) died within 3 months; 3) other malignancies. The baseline organ recipients' demographic characteristics including age, sex, and transplant outcomes including cancer‐related mortality. For groups, we constructed Kaplan‐Meier curves to estimate overall survival proportions and cumulative incidence curves. All recipients received tacrolimus in their initial immunosuppressant regimen, and part of those received sirolimus in the long term regimen.
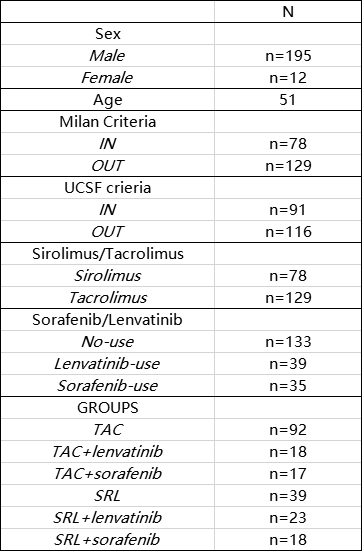
Results and Discussion: Between 2014 and 2019, 207 liver recipients remained in our study population with a follow‐up starting 3 months after transplantation (maximum follow‐up of 5 years). Among these recipients, 78 (37.7%) received SRL in their long-term immunosuppressant regimen, 71(34.3%) received lenvatinib and most of recipients are beyond Milan criteria. In total, there were 69 recurrences and 32 cancer-related deaths. HCC recurrence rates was similar in sirolimus users and non-users in the In-Milan criteria, while appeared lower in sirolimus users compared with tacrolimus users in the Out-Milan criteria group. All recipients were clsssifed into six groups (SRL+ lenvatinib, TAC+ lenvatinib, SRL+ sorafenib, TAC+ sorafenib, SRL only, TAC only). The results show that especially in Out-Milan group, patients were benefied from TAC combined with lenvatinib effectively. Targeted therapies combined with sirolimus may bring a better prognosis among patients who do not meet the Milan criteria. Partly because of the limited data, the best combination of immunosuppression regimen and immunotherapies for different risky groups needs further study to be verified.
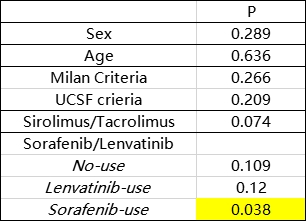
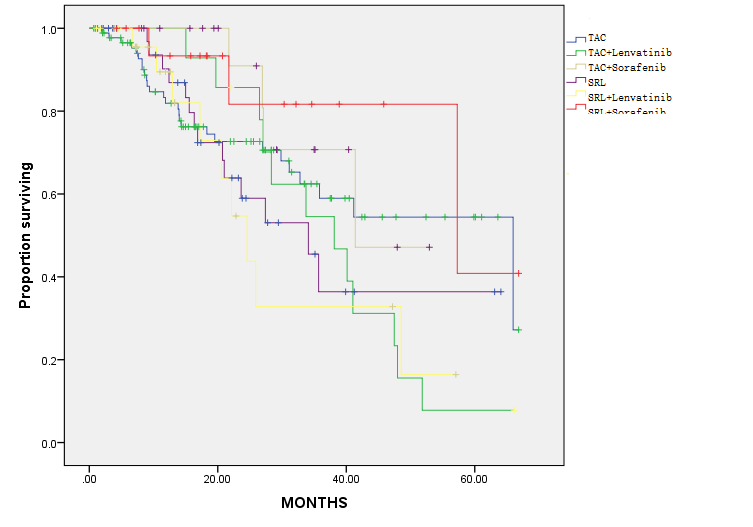
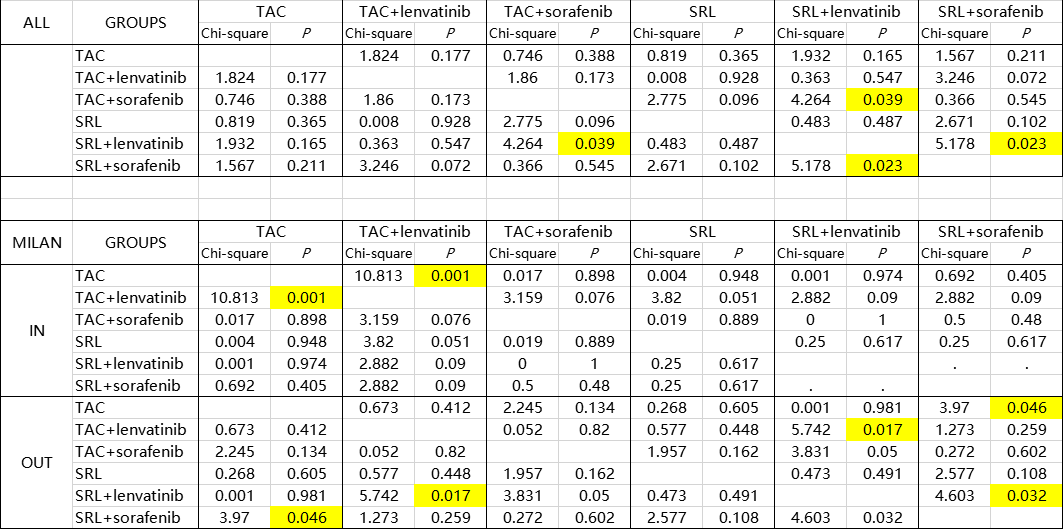
Conclusion: In conclusion, immunosuppression and immune-targeted durgs are feasible measures for preventing HCC recurrence of recipients for HCC-transplantation. Intervention like modulating dose of SRL/TAC and sorafenib/lenvatinib may help patients prevent recurrence and receive a better long-term prognosis.
[1] 1. Yanik EL, Chinnakotla S, Gustafson SK, et al. Effects of maintenance immunosuppression with sirolimus after liver transplant for hepatocellular carcinoma. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2016; 22 (5): 627.
[2] 2. Verna EC, Patel YA, Aggarwal A, et al. Liver transplantation for hepatocellular carcinoma: Management after thetransplant. American journal of transplantation : official journal of the American Society ofTransplantation and the American Society of Transplant Surgeons 2020; 20 (2): 333.
[3] 3. Grigg SE, Sarri GL, Gow PJ, Yeomans ND. Systematic review with meta-analysis: sirolimus- or everolimus-basedimmunosuppression following liver transplantation for hepatocellular carcinoma. Alimentary pharmacology & therapeutics 2019; 49 (10): 1260.
[4] 4. Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in livertransplantation for hepatocellular carcinoma. Alimentary pharmacology & therapeutics 2013; 37 (4): 411.
[5] 5. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England) 2018; 391 (10127): 1301.
There are no comments yet...