
Efficacy and safety of everolimus with reduced-exposure cyclosporine in de novo kidney transplant recipients: 24-month analysis from the TRANSFORM study
Claudia Sommerer1, Aleksey Pinchuk2, Silvina Aleman3, Vincent Vuiblet4, Van der Giet5, Angel Joaquin Amante6, Duck Jong Han7, Uyen Huynh-Do8, Atiporn Ingsathit9, Apurva Gawai10, Maria Pilar Hernandez Gutierrez11, Mario Carmellini12.
1Department of Nephrology, University Hospital, Renal Clinic , Heidelberg, Germany; 2SRI of emergency care , Sklifosovsky Institute, Moscow, Russian Federation; 3Departament of Kidney Transplant, Hospital CUCAIBA-CRAI Norte, Buenos Aires , Argentina; 4Nephrology Department, CHU de Reims, Reims, France; 5Med. Klinik mit SP Nephrologie und Internistischer Intensivmedizin, Universitätsmedizin Berlin, Berlin, Germany; 6Department of Adult Nephrology, National Kidney and Transplant Institute, Quezon City, Philippines; 7Department of Surgery, Asan Medical Center, Seoul, Korea; 8Division of Nephrology & Hypertension, University Hospital Bern, Bern, Switzerland; 9Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 10Novartis India Limited, Mumbai, India; 11Department of Research and Development, Novartis Pharma AG, Basel, Switzerland; 12Department of Medical, Surgical and Neurologic Sciences, University of Siena, Siena, Italy
Background: TRANSFORM is the largest study until now in de novo kidney transplant recipients (KTxR) receiving either everolimus (EVR) with reduced-dose calcineurin inhibitor (rCNI; tacrolimus [TAC] or cyclosporine [CsA]) or mycophenolic acid (MPA) with standard-dose (s) CNI. Here we present the efficacy and safety data from patients in the study receiving CsA.
Methods: TRANSFORM was a 24-month, prospective, open-label trial in 2037 KTxRs randomized from across 42 countries. At Month (M) 24, the primary endpoint of treated biopsy proven acute rejection (tBPAR) or estimated glomerular filtration rate (eGFR) <50 mL/min/1.73 m2 and the key secondary endpoints including composite efficacy failure (tBPAR, graft loss and death), evolution of renal function and safety were evaluated.
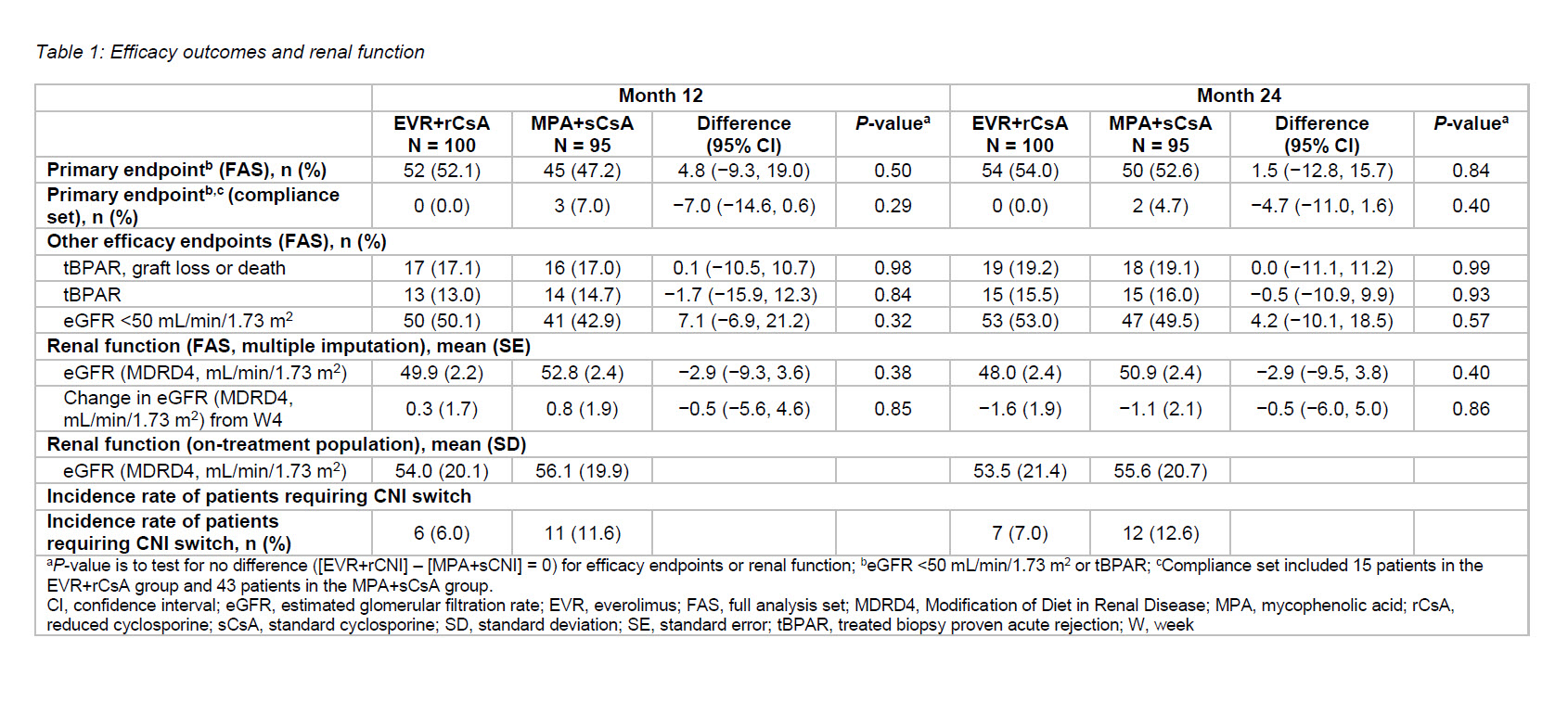
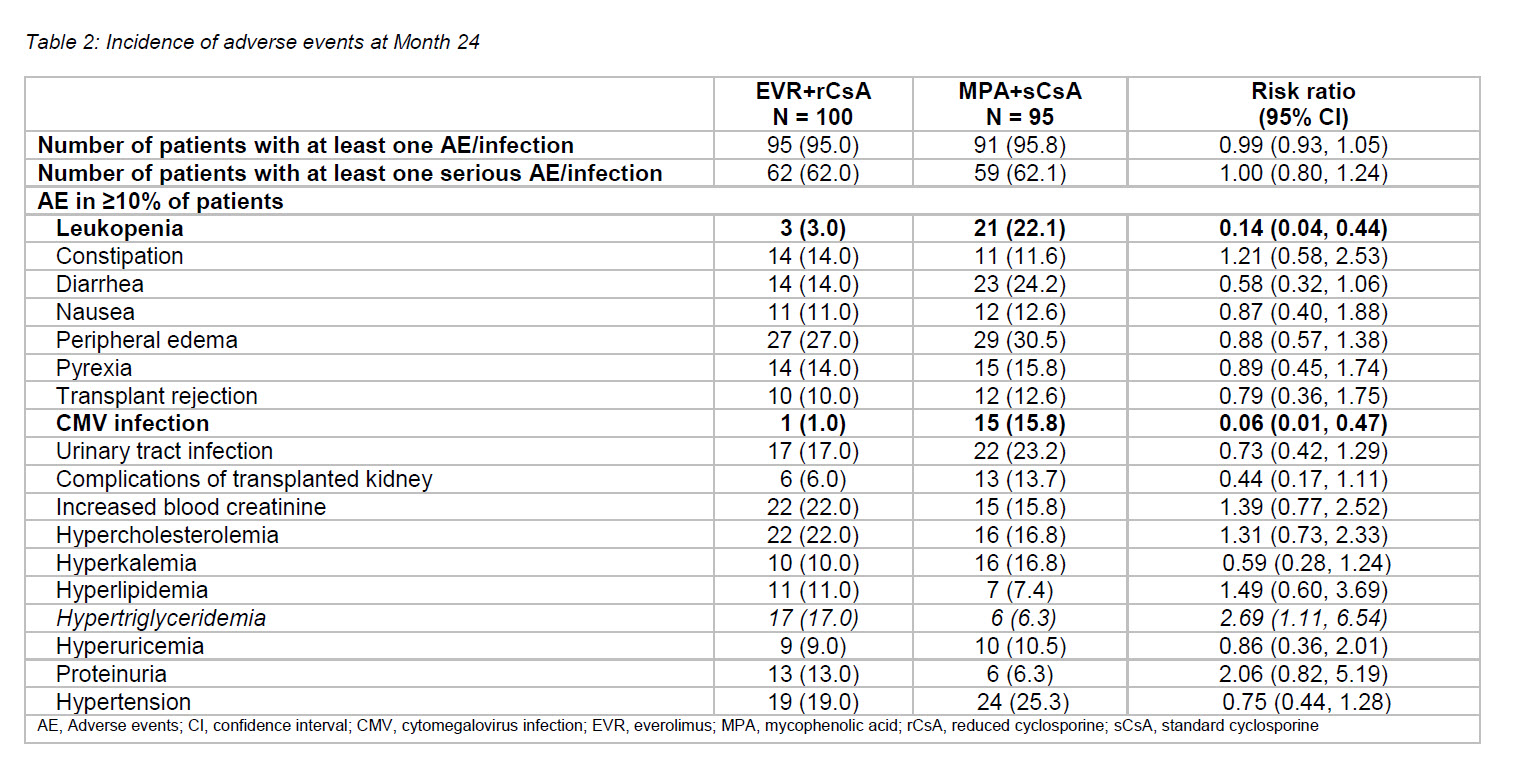
Results: Approximately 9% of the total randomized patients received cyclosporine (EVR+rCsA, n = 100; 9.9% and MPA+sCsA, n = 95; 9.4%), of which 69% and 80% patients completed study medication in the EVR+rCsA and MPA+sCsA arms, respectively. Mean CsA trough levels were above target range in up to 61.3% of patients in the EVR+rCsA arm versus 16.7% of patients in the MPA+sCsA arm. A higher proportion of patients receiving MPA+sCsA (12.6%) required CNI switch compared to those receiving EVR+rCsA (7.0%). Overall, 15 and 43 patients were compliant to the treatment regimen in the EVR+rCsA and MPA+sCsA arms, respectively. At M24, the incidence of primary endpoint and composite efficacy failure was comparable between arms (Table 1) in the full analysis set (FAS). Among patients compliant with treatment regimens, incidence of the primary endpoint at M24 was 0.0% and 4.7% in the EVR+rCsA and MPA+sCsA arms, respectively. Evolution of renal function was similar between arms; however, it was slightly better in both arms among on-treatment patients relative to FAS (Table 1). Incidence of adverse events (AEs) and serious AEs were comparable between both treatments. Leukopenia was significantly more frequent in the MPA+sCsA arm and hypertriglyceridemia was more frequent in the EVR+rCsA arm (Table 2). Cytomegalovirus (CMV) infection rates were significantly lower with EVR+rCsA (risk ratio [95%CI]: 0.06 [0.01, 0.47]), and incidence of BKV was comparable between EVR+rCsA (6.0%) and MPA+sCsA (5.3%) at 24 months.
Discussions: The between-treatment differences in incidence of primary endpoint (1.5% vs 4.2%) and tBPAR (−0.5% vs 0.7%) were lower in the CsA-receiving patients versus the overall population1, respectively. In the compliance set, a treatment difference of −4.7% for the primary endpoint was seen in the CsA subset versus −3.7% in the overall population. Safety profile was also similar to that observed in the overall population.
Conclusions: De novo EVR+rCsA offers comparable efficacy and graft function and significantly lower incidence of CMV infections compared with an MPA+sCsA up to 24 months.
[1] Berger S et.al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from TRANSFORM study. Am J Transplant. 2019; 19:3018–3034
There are no comments yet...