Athena vs Hephaistos - Are discontinuation rates in multicenter studies driven by facts or attitudes?
Björn Nashan1,2, Felix Braun2, Duska Dragun1, Ingeborg A. Hauser1, Peter Schemmer2, Hans J. Schlitt2, Claudia Sommerer1, Barbara Suwelack1, Christiane Schiedel3, Irena Kroeger3, Friedrich Thaiss1.
1Athena, Study, Group, Germany; 2Hephaistos, Study, Group, Germany; 3Novartis Pharma, GmbH, Nürnberg, Germany
Introduction: The aim of both, the HEPHAISTOS [NCT01551212] and ATHENA [NCT01843348] study, was to compare everolimus [EVR] combined with reduced tacrolimus [rTAC] vs. TAC alone in patients [pts] after de novo liver transplantation [LTx], or EVR combined with reduced cyclosporine A [rCyA] or rTAC vs. a conventional treatment regimen consisting of mycophenolic acid [MPA] plus TAC in patients after de novo kidney transplantation [KTx], respectively. Here, discontinuation rates of these two trials were explored, as it has often been presumed that treatment discontinuation due to adverse events [AEs] is higher with EVR than with standard regimens.
Materials and Methods: HEPHAISTOS and ATHENA were two 12-months [M] open-label, prospective multicenter studies. For HEPHAISTOS, 333 pts were included and randomized to EVR/rTAC or TAC alone between day 7-21 after LTx, all with steroids until M6. In ATHENA a total of 612 pts was randomized at the time of KTx to EVR/rTAC, EVR/rCyA or TAC/MPA in Germany and France.
Results and Discussion: Table 1 displays the adjustment and discontinuation rates of both studies. Generally, no AE-specific correlation concerning adjustment or discontinuation was seen, indicating that there were no specific drug-related reasons for either. Interestingly, in both studies the total discontinuation rates occurred in an opposing manner between EVR and control groups.
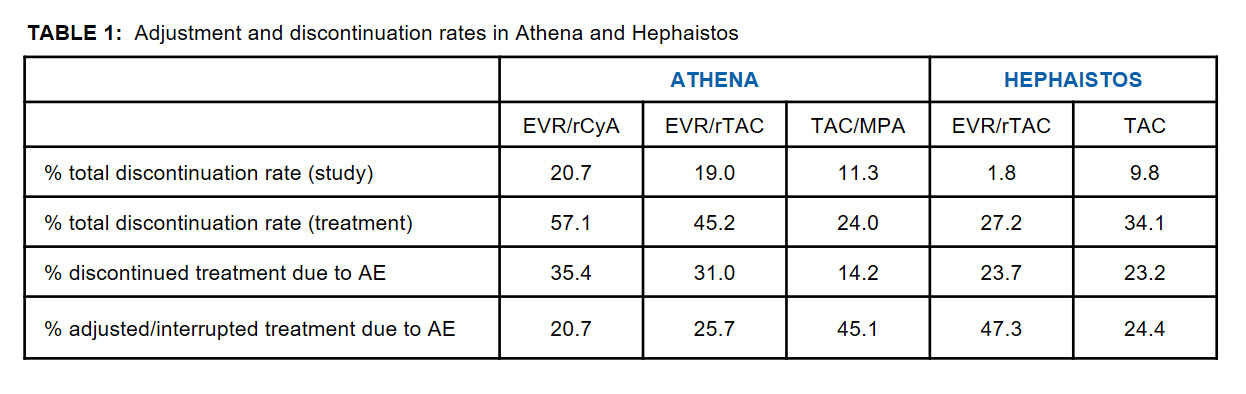
Furthermore, variations in study drug discontinuation rates between centers were lower in HEPHAISTOS (0-53% EVR/TAC and 0-62% TAC group), than in ATHENA (0-100%, 0-100% and 0-60% in the EVR/rCyA, EVR/rTAC and TAC/MPA groups, respectively).
Conclusion: HEPHAISTOS and ATHENA both confirmed safety and efficacy of EVR with rCyA or rTAC in both LTx and KTx. A new and remarkable finding of this analysis was the contrary occurrence of discontinuation rates in treatment vs. control groups between LTx and KTx. Further, no drug-related reasons for discontinuation could be identified based on the AE analysis, but significant differences between organs and centers were evident. Although as of now we have no concrete explanation for these interesting results, we hypothesize that investigators’ experience- and/or background-driven attitudes might play a part and could help in understanding these findings as well as differences observed in other multicenter clinical studies in general.
[1] Nashan et al. Trials. 2015; 16:118
[2] Sommerer et al. Kidney International. 2019; 96, 1: 231-244
There are no comments yet...