Simultaneously detection of ddcfDNA in plasma and urine assist to determine the type of rejection in renal transplant patients
Cheng Dongrui1, Feng Liu2, Liu Haitao2, Jiang Tingya2, Zhou Yang3, Shu Liping2, Li Xue1, Xie Kenan1, Ni Xuefeng1, Chen Jingsong1, Ge Jun2, Liu Zhihong1.
1Jinling Hospital, Nanjing University, Nanjing, People's Republic of China; 2Market Department, AlloDx, Shanghai, People's Republic of China; 3 Institute of Life Science, Jiangsu University, Zhenjiang, People's Republic of China
Background: The correct diagnosis and treatment of acute rejection (ABMR and TCMR) are the key to the long-term survival after renal transplantation. Renal biopsy is a gold standard for diagnosis of ABMR and TCMR. Donor-derived cell-free DNA (ddcfDNA) detected in transplant recipients has been reported as a noninvasive marker to diagnose allograft injury. Pathological analysis show that ABMR and TCMR have different injury sites for the graft, the ABMR mainly infects vascular endothelial cells and apoptotic endothelial cells release cfDNA into blood stream directly. Meanwhile, TCMR-associated injury that principally occurs in the tubulointerstitial compartment and apoptotic epithelial cells release cfDNA into urine directly. Herein, the purpose of this study was to perform ddcfDNA analysis of blood and urine to determine the type of rejection in renal transplant patients.
Methods: DdcfDNA was assayed in plasma and corresponding urine samples from 56 recipients whose renal biopsy proved acute rejection. We measured the fraction of ddcfDNA in plasma and the absolute amount of ddcfDNA in urine (in-house data show absolute quantity of ddcfDNA have more significant in urine). ddcfDNA(%) quantification through target region capture sequencing and calculated by maximum likehood estimation (MLE).
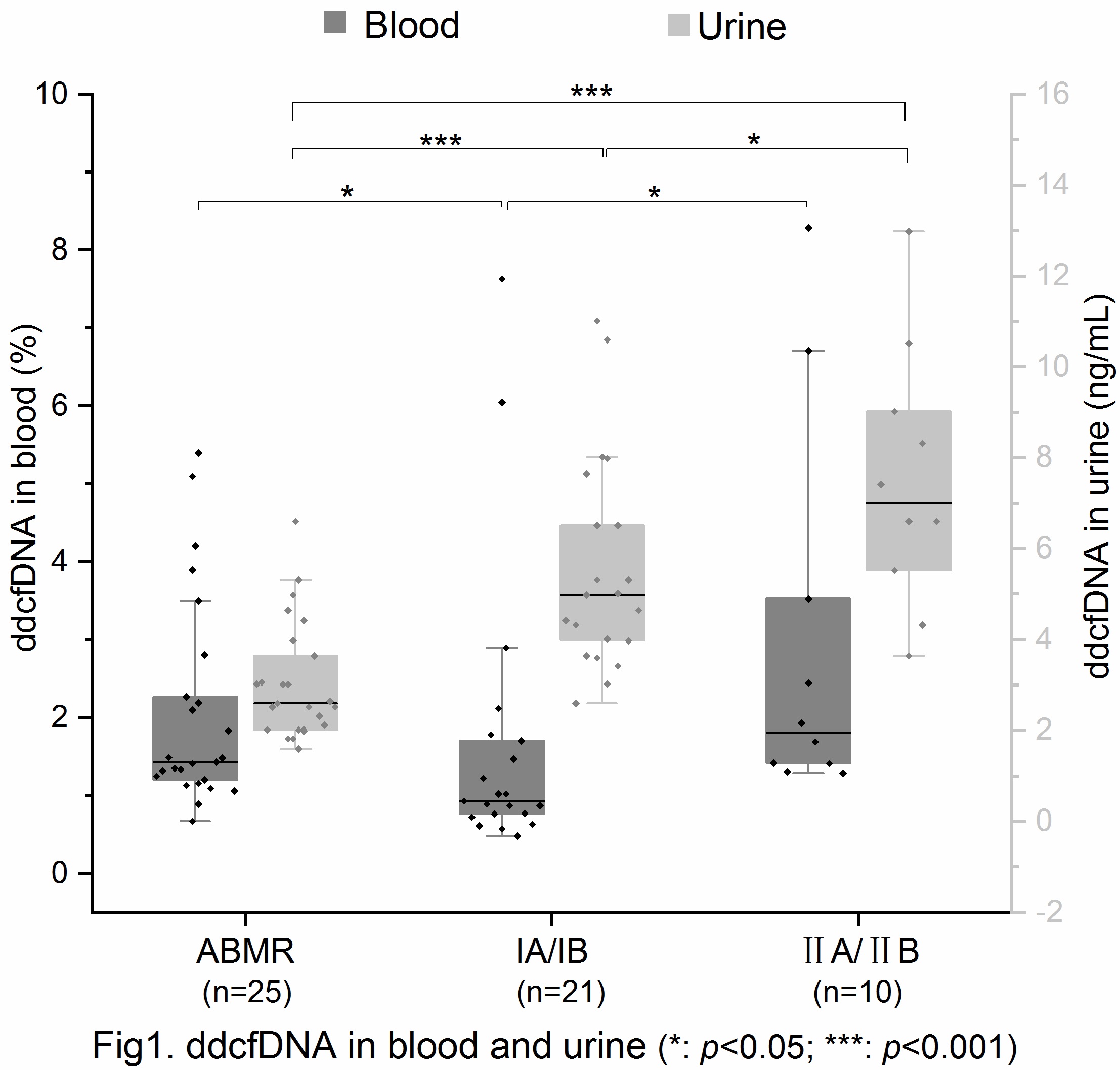
Results: Allograft pathology was classified according to the Banff 2013, including 25 patients with biopsy-proven ABMR, 21 patients with TCMR IA/IB and 10 patients with TCMR IIA/IIB. All samples have no BKV detected in urine or blood. Analysis ddcfDNA in plasma , the median ddcfDNA level of ABMR group was 1.43% (IQR 1.18%-2.54%), TCMR IA/IB group was 0.93%(IQR 0.74%-1.74%), TCMR IIA/IIB group was 1.81% (IQR 1.39%-4.33%). Significant differences between IA/IB group and ABMR group (p=0.013)/IIA/IIB group(p=0.01) was found, but no significant difference between ABMR group and IIA/IIB group (p>0.05). Analysis ddcfDNA in urine, the median ddcfDNA(ng/mL) of ABMR, TCMR IA/IB and TCMR IIA/IIB group was 2.6ng/mL (IQR 2.02ng/mL-3.82ng/mL), 4.98ng/mL (IQR 3.82ng/mL-7.09ng/mL), 7.01ng/mL (IQR 5.22ng/mL-9.39ng/mL), respectively. There were significant differences among the three groups (p<0.001; p<0.001; p=0.047).
Conclusions: Our results indicated that simultaneous detection ddcfDNA in blood and urine can distinguish the type of rejection of renal transplantation (ABMR, TCMR I/II).