Pregnancy outcomes in 331 female liver transplant recipients
Lisa Coscia1, Theresa Daly1, Howard M. Nathan2, Richard D. Hasz2, Serban Constantinescu1,3, Michael J. Moritz1,4,5.
1Transplant Pregnancy Registry International, Gift of Life Institute, Philadelphia, PA, United States; 2Gift of Life Donor Program, Philadelphia, PA, United States; 3Medicine, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, United States; 4Surgery, Lehigh Valley Health Network, Allentown, PA, United States; 5Surgery, Morsani College of Medicine, Tampa, FL, United States
The purpose of this study is to describe 651 pregnancies in 331 liver transplant recipients reporting to the Transplant Pregnancy Registry International (TPR) from 1991 to 2019. Data were collected via questionnaires, telephone interviews, and medical records review. Data are in the table below. There were 26 recipients received a living donor liver transplant prior to their pregnancy.
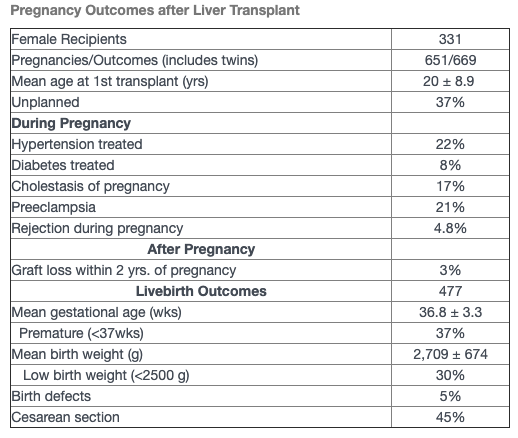
The majority of recipients were on calcineurin inhibitor therapy for immunosuppression, 34% on cyclosporine and 63% on tacrolimus; 6.7% of pregnancies were also exposed to a mycophenolic acid product (MPA) during the first trimester. There were a total of 669 pregnancy outcomes (including multiples) resulting in 477 live births (71%), miscarriages (24%), terminations (3%), stillbirths (1%) and ectopic pregnancies (1%). There were 43 1st trimester exposures to MPA resulting in 1 termination, 2 stillbirths, 25 miscarriages, 15 live births (3 with birth defects). There was a two-fold increase in reporting of fertility issues (22%) among recipients compared to the general population (11%) and 11 recipients conceived via in vitro fertilization. Cholestasis of pregnancy occurred in 17% (51/301) of pregnancies which was a much higher incidence than the general population which is 1%. Live birth outcomes are listed in the table. There were 135 children breastfed. The TPR has been following the offspring long-term: mean age of children 9 ± 7.6 yrs. With a last mean maternal follow-up of 10.5 ± 13.6 years, 43 recipients died, 12 reported reduced function, 9 unknown, and 267 reported adequate graft function.
Conclusion: Female liver transplant recipients have reported successful pregnancies, with a live birth rate of 71%, and low incidence of rejection during pregnancy (4.8%). There is an increased risk of premature and low birthweight infants. Cholestasis of pregnancy is much more common in this population and requires additional investigation. The risk of miscarriage and birth defects when pregnancies are exposed to MPA in utero underscores the need for pre-conception counseling. Infertility in liver transplant recipients requires additional study. All centers worldwide are encouraged to have their recipients report pregnancies to the TPR.
[1] Transplant Pregnancy Registry International (TPR) 2018 Annual Report, Gift of Life Institute, Philadelphia, PA 2019.
[2] Sarkar M, Bramham K, Moritz M, Coscia L. Reproductive Health in Women Following Abdominal Organ Transplant. Am J Transplant. 2018;18(5):1068-1076.