PD-L1 / PD-1 interactions are critical for tolerance induction in primed skin graft recipients following liver-directed MHC class I gene transfer
Mario Leong1, Moumita Paul-Heng1, Eric T. Son1, Markus J. Hofer2, Chuanmin Wang1, Patrick Bertolino3, Alex Bishop1, David Bowen1,3, Alexandra F Sharland1.
1Transplantation Immunobiology Group, Central Clinical School, Faculty of Medicine and Health, , University of Sydney, Sydney, Australia; 2School of Life and Environmental Sciences, University of Sydney, Sydney, Australia; 3AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital and University of Sydney, Sydney, Australia
Background: Transduction of C57BL/6 recipient hepatocytes with AAV vectors encoding donor MHC class I results in tolerance to subsequent skin grafts expressing the same mismatched allomorph. C57BL/6 (H-2b) CD8+ T cells encountering H-2Kd in the liver express high levels of PD-1 1. We aimed to determine whether the PD-L1/PD-1 axis contributes to resolution of inflammation and tolerance induction in naïve and primed recipients.
Methods: Naïve or primed male C57BL/6 or PD-L1 KO mice (both H-2b) were inoculated with AAV-Kd 5x1011 vgc and samples collected between d7 and d28 post inoculation for serum biochemistry, immunostaining and flow cytometry. Other naïve and primed mice were challenged with B6.Kd skin grafts d7 post inoculation with AAV-Kd.
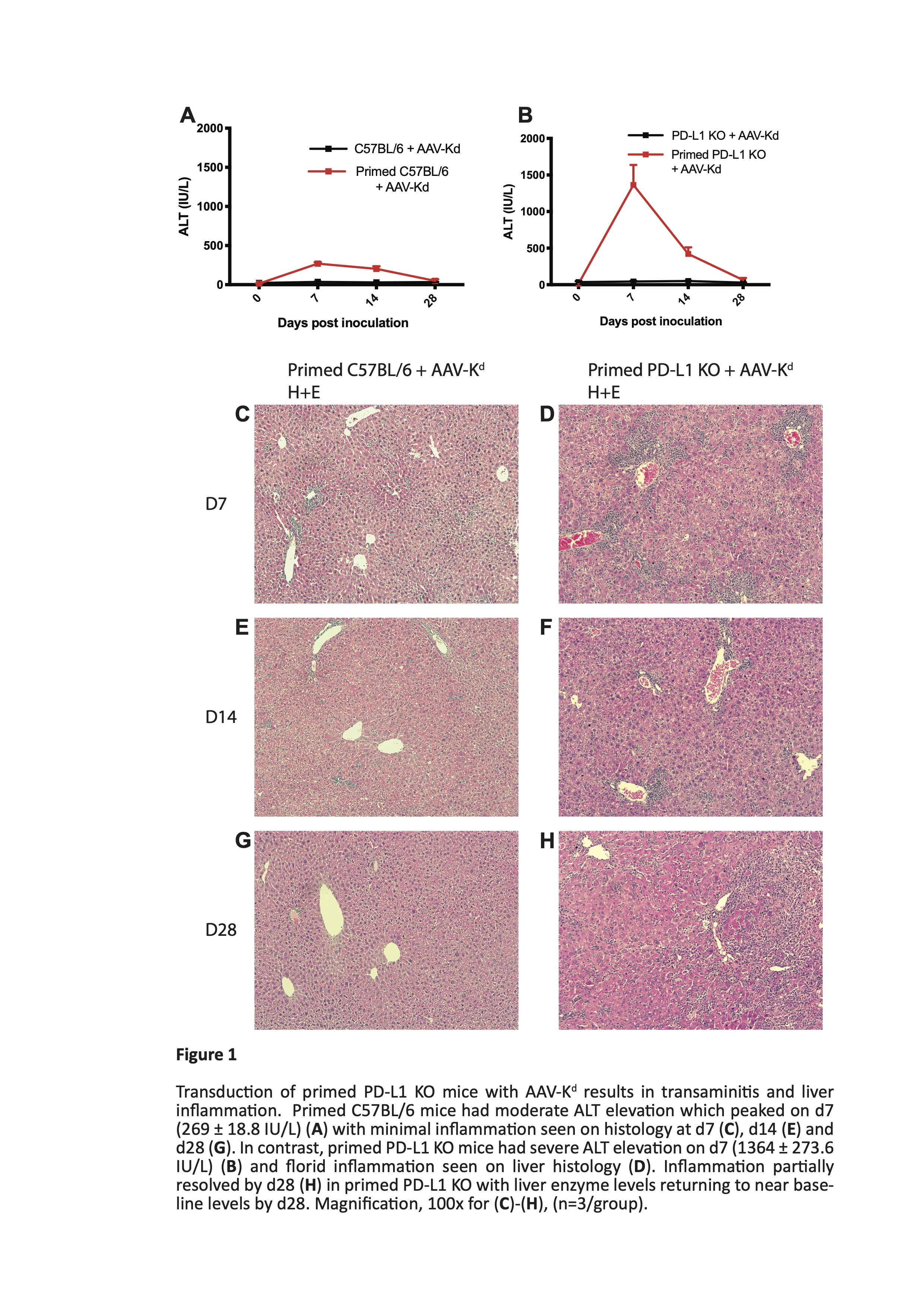
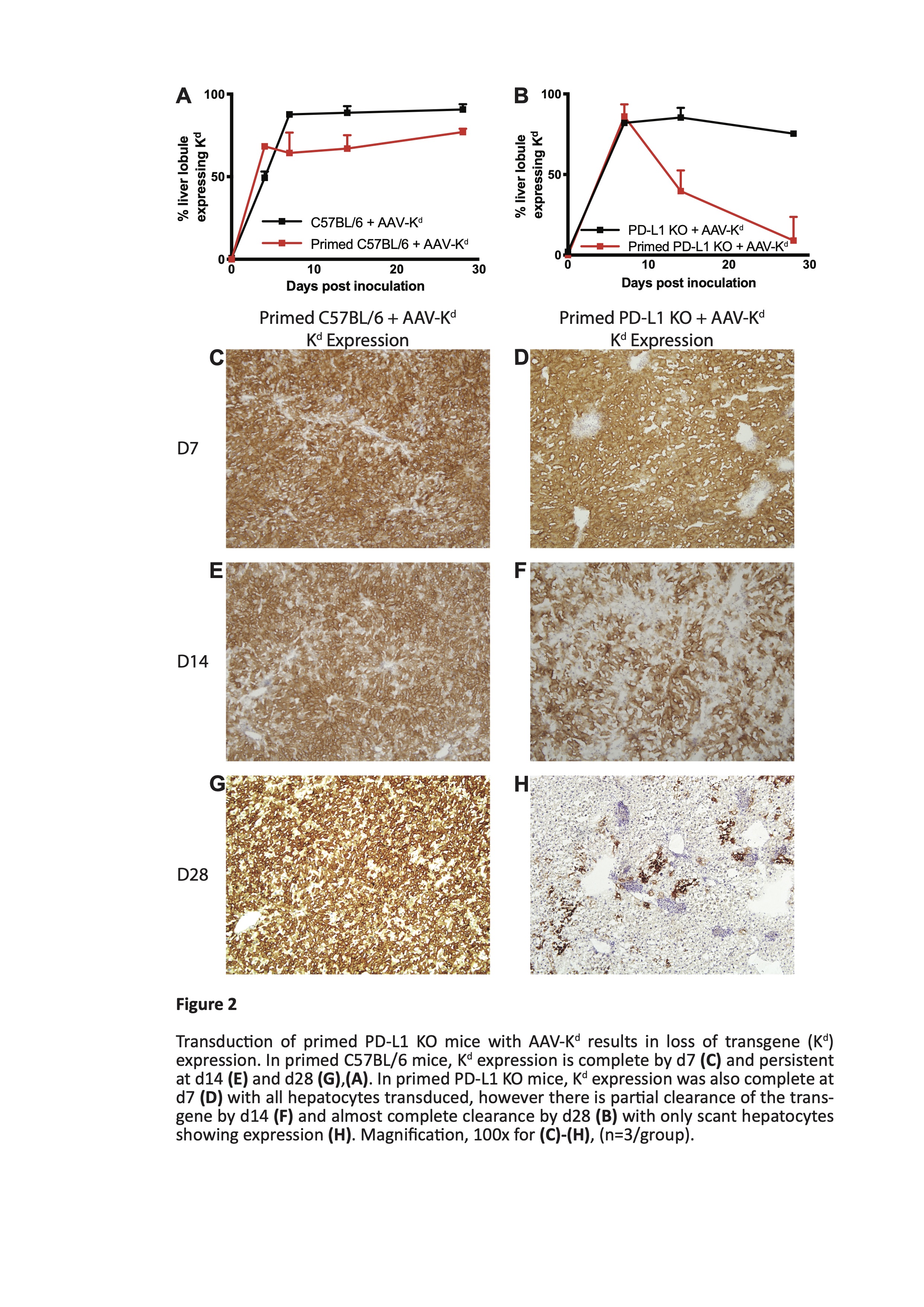
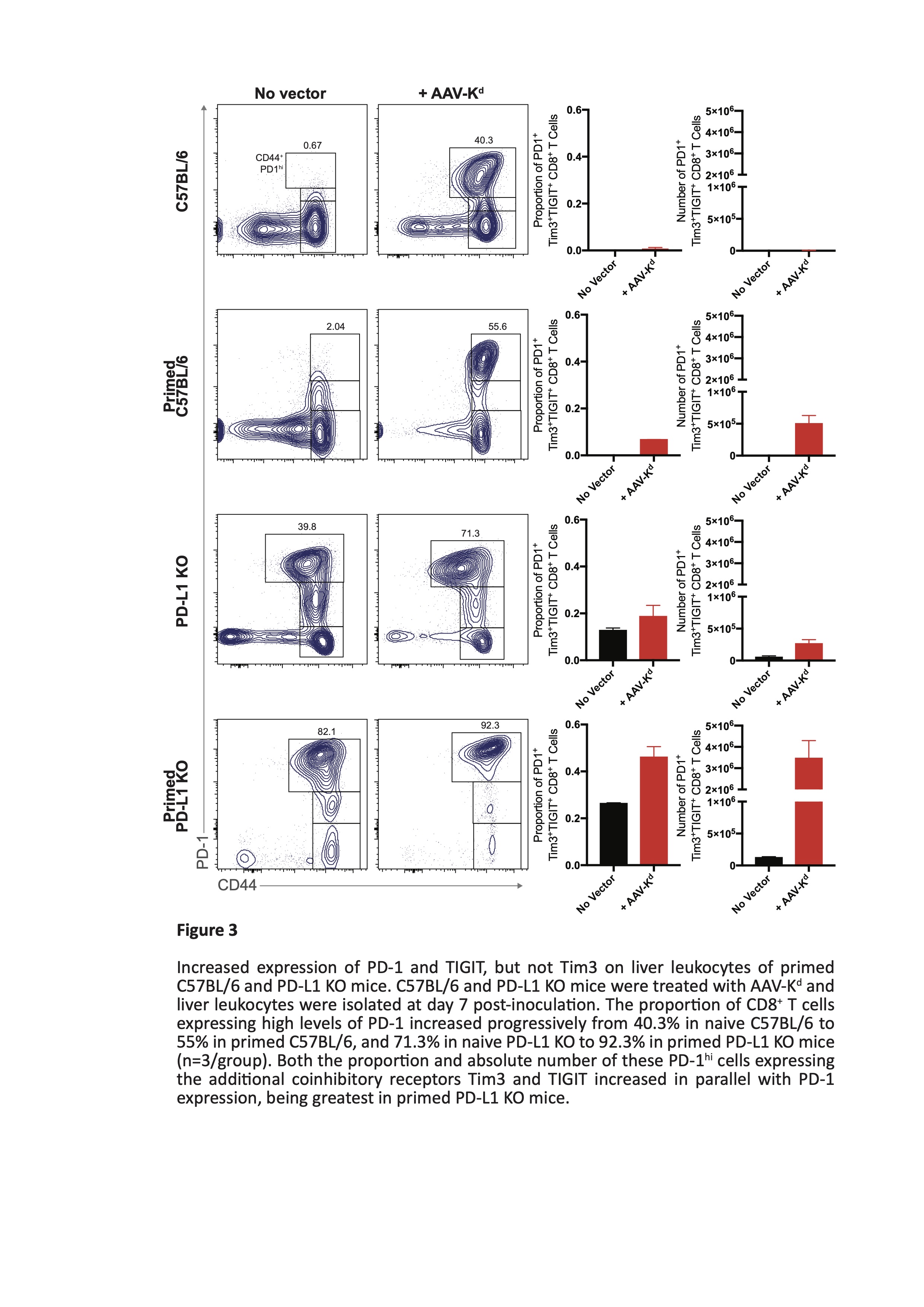
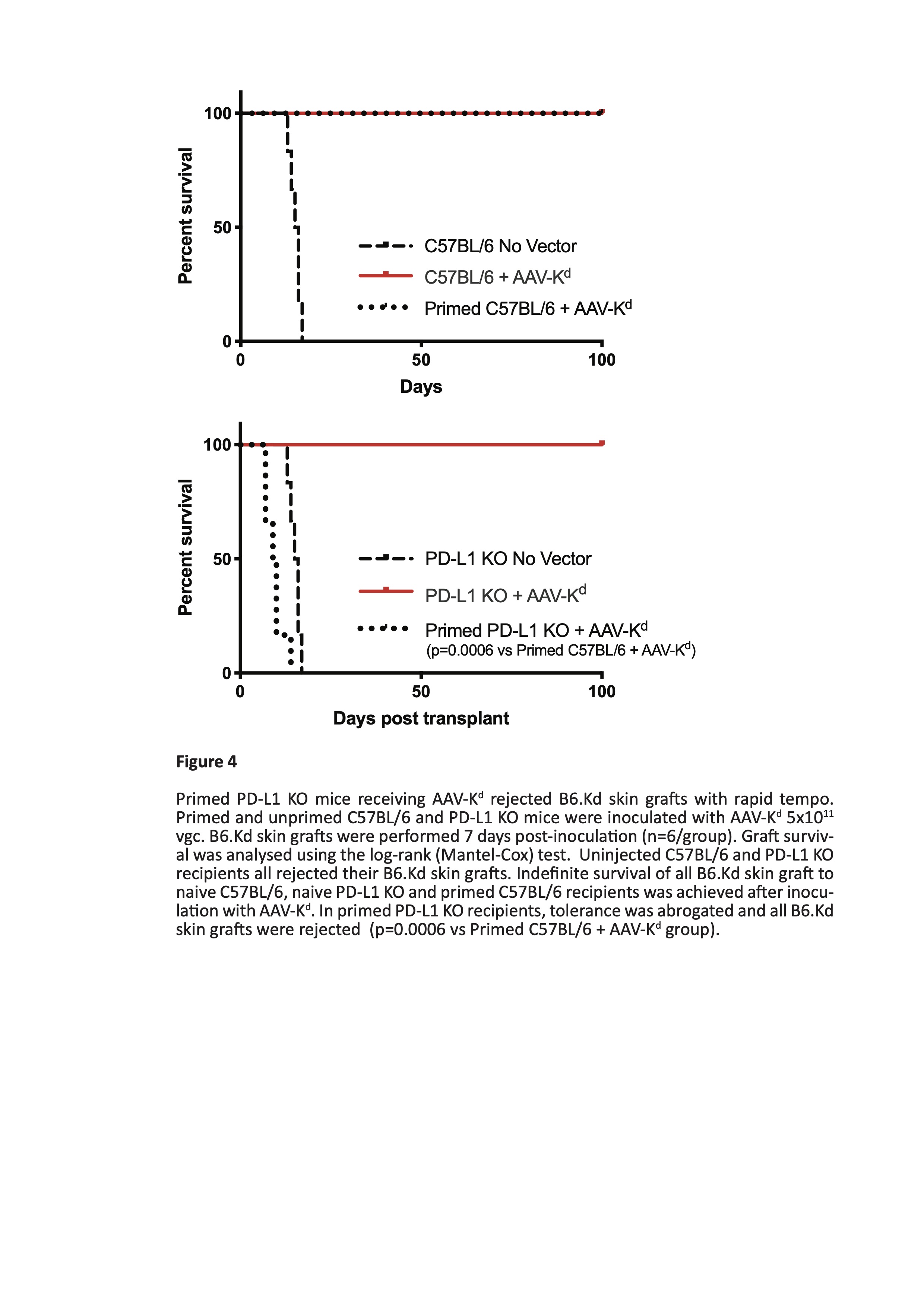
Results: Liver ALT levels remained within the normal range in AAV-Kd-treated naïve C57BL/6 and PD-L1 KO mice. Liver morphology was normal and Kd expression was near maximal by d7 persisting though d28 post inoculation. In primed C57BL/6 mice ALT peaked on d7 (269 ± 18.8 IU/L) before returning to baseline levels by d28. Numbers of both CD4+ T cells (262 ± 59.5 cells/hpf) and CD8+ T cells (142.3 ± 29.6 cells /hpf) peaked on d14. Kd expression reflected that in naïve mice. In contrast, severe and widespread inflammation was seen in primed PD-L1 deficient mice. ALT levels reached a maximum on d7 (1364 ± 273.6 IU/L) (figure 1), accompanied by marked elevation of CD4+ T cells (725 ± 250.3 cells/hpf) and moderate increase in CD8+ T cells (157 ± 29.1 cells/hpf). Kd expression was rapidly lost from almost all hepatocytes by d28 post inoculation (figure 2). Isolated liver leucocytes were characterized by flow cytometry. A mixed infiltrate comprising CD4+ and CD8+ T cells, B cells, NK cells, and myeloid cells was present. CD8+ T cells were predominantly of tissue-resident memory phenotype. Of note, over 90% of CD8+ T cells expressed high levels of PD-1, and of these, 72 ± 2.3% were also positive for both Tim-3 and TIGIT (figure 3). Few cells expressed either LAG-3 or CTLA-4. Tolerance to B6.Kd skin grafts was achieved in all AAV-Kd treated naïve C57BL/6 and PD-L1 KO mice, as well as in C57BL/6 mice primed by rejection of a prior Kd-bearing skin graft. Without AAV-Kd treatment, C57BL/6 and PD-L1 KO mice all rejected B6.Kd skin grafts with a similar tempo (MST 15.5 and 12.0 days respectively). Primed PD-L1 KO mice receiving AAV-Kd rejected B6.Kd skin grafts with rapid tempo (MST 9.5 days, p=0.0006 vs primed C57BL/6 treated with AAV-Kd) (figure 4).
Conclusions: The PD-L1/PD-1 axis is dispensible for control of inflammation and tolerance induction after AAV-Kd inoculation in naïve mice, presumably because of the redundancy of co-inhibitory pathways. Conversely, despite strong expression of the receptors Tim-3 and TIGIT in addition to PD-1, the Kdtransgene is rapidly lost in primed PD-L1 deficient mice due to a florid inflammatory response in the liver, and tolerance to secondary grafts cannot be achieved.
[1] Paul-Heng M, Leong M, Cunningham E, Bunker DLJ, Bremner K, Wang Z, et al. Direct recognition of hepatocyte-expressed MHC class I alloantigens is required for tolerance induction. JCI Insight., 2018;3(15).