Discovery of pMHC epitopes for directly alloreactive T cells
Eric Taeyoung Son1, Moumita Paul-Heng1, Mario Leong1, Pouya Faridi2, Nadine Dudek2, Ian Alexander3,4, Patrick Bertolino5, Anthony Purcell2, David Bowen1,5, Nicole Mifsud2, Alexandra Sharland1.
1Central Clinical School, The University of Sydney, Sydney, Australia; 2Department of Biochemistry and Molecular Biology, Monash University, Melbourne, Australia; 3Gene Therapy Research Unit, Children's Medical Research Institute, Sydney, Australia; 4Discipline of Child and Adolescent Health, The University of Sydney, Sydney, Australia; 5AW Morrow Gastroenterology & Liver Centre, Royal Prince Alfred Hospital and The University of Sydney, Sydney, Australia
Introduction and Aims: Expression of donor MHC class I molecules (MHC-I) in recipient hepatocytes induces tolerance to subsequent allogeneic skin grafts. Direct allorecognition of intact donor MHC-I molecules is required for tolerance induction. Few peptide/MHC (pMHC) epitopes recognized by alloreactive CD8+ T cells are known. We examined the self-immunopeptidome for potential pMHC targets of alloreactive T cells.
Methods: We excluded donor MHC-I presentation of endogenous peptides using an AAV vector construct expressing donor heavy chain, b2m and a defined peptide as a single-chain trimer. Global alterations in the bound peptide repertoire were introduced using mutant MHC-I molecules (Kd-YCAC), which are stable in the presence of low-affinity peptides or even when empty, and recipient mice with a hepatocyte-specific deficiency of Tap1. Mice were then challenged with allogeneic skin grafts.
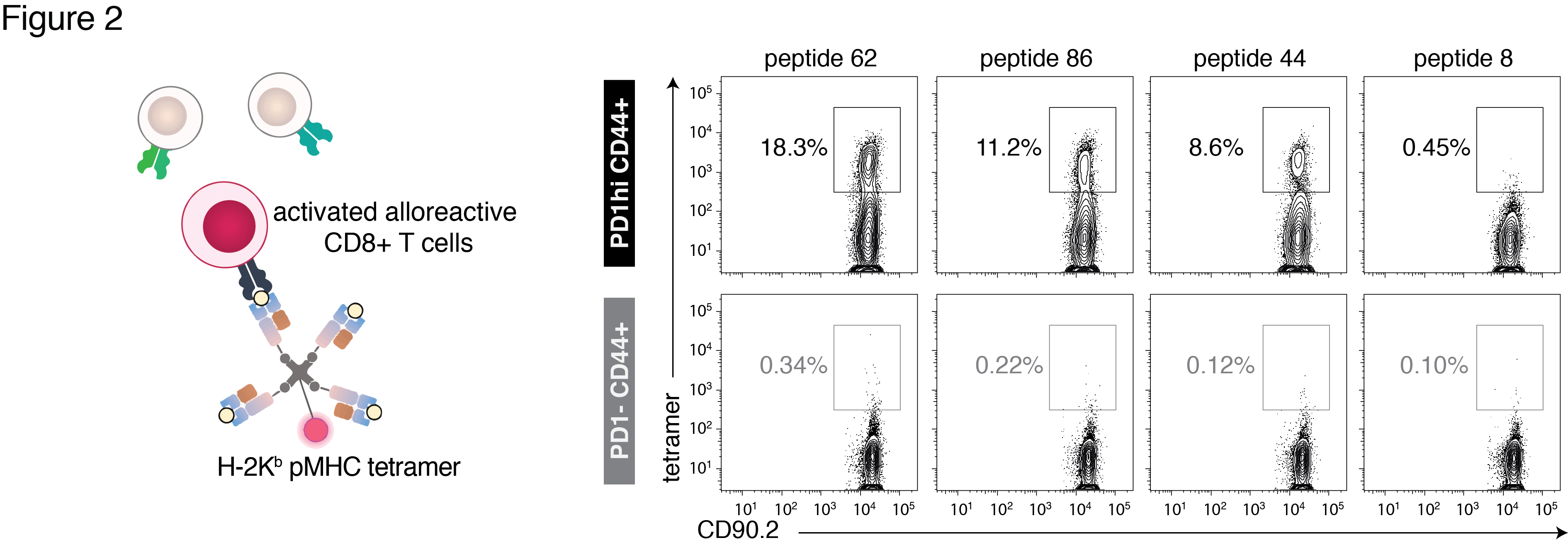
To identify the pMHC epitopes recognized by alloreactive T cells, the H-2Kb peptide repertoire was determined using immunoaffinity purification, RP-HPLC and LC-tandem mass spectrometry. H-2Kb-reactive CD8+ T cells were screened using pMHC tetramer staining. Simultaneous staining with two different pMHC tetramers was used to evaluate the proportions of T cells recognizing more than one pMHC specificity.
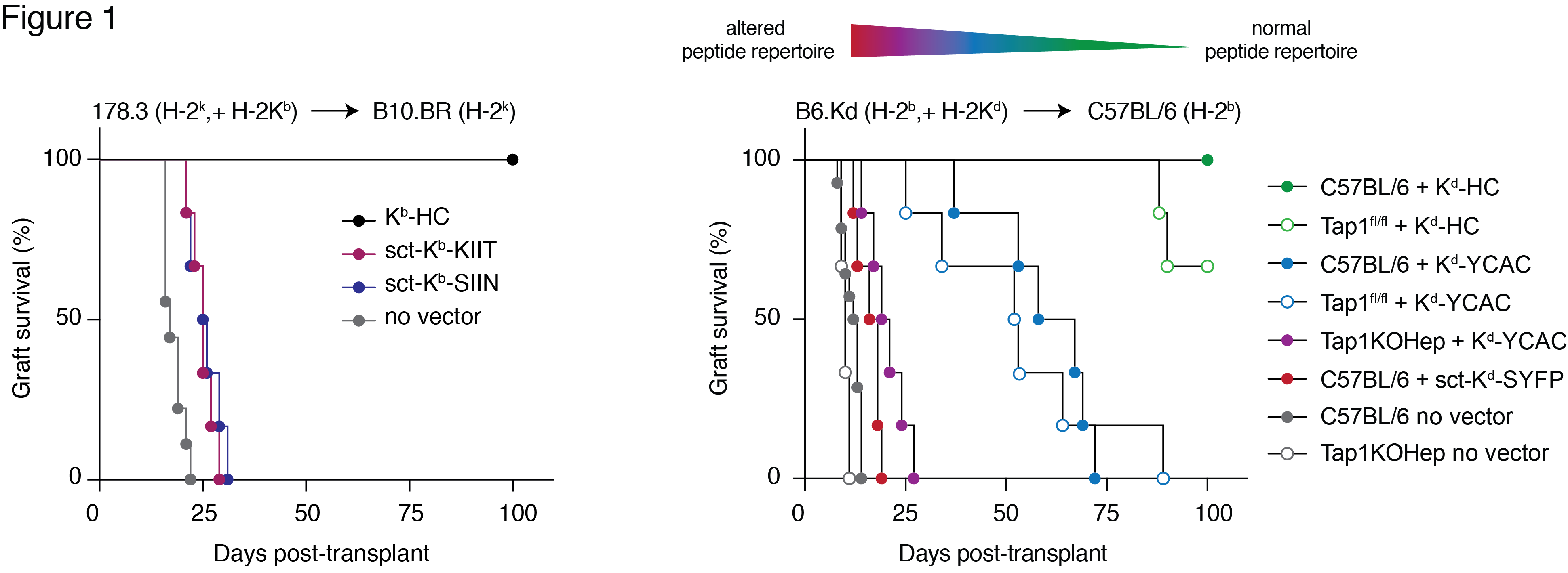
Results: Expression of a single donor pMHC in recipient liver prevented skin graft rejection that was mediated by a T cell clone bearing the cognate antigen receptor. Conversely, skin graft tolerance in recipient mice with a polyclonal repertoire of responding T cells was only achieved when donor MHC-I was able to present the self-immunopeptidome. Progressive shortening of graft survival accompanied increasing disruption of the immunopeptidome of recipient hepatocytes (Fig 1).
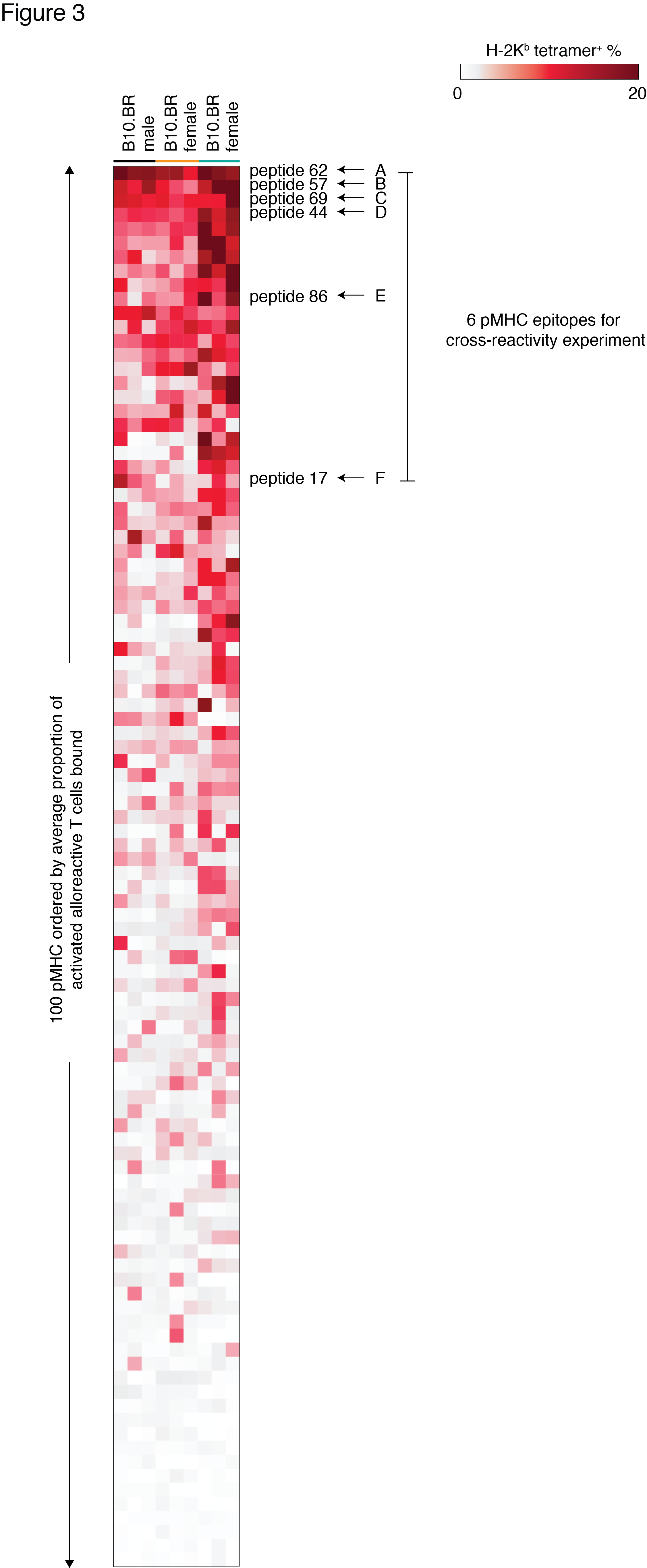
332 unique H2-Kb-bound peptides were shared by multiple tissues. Of 100 peptides screened, 17 bound greater than 5% of alloreactive T cells from male B10.BR (Figs 2-3). pMHC epitopes recognized by male B10.BR mice were also recognized by female B10.BR (R=0.76, p<0.0001) and male BALB/c (H-2d) (R=0.62, p<0.0001) (Fig 3).
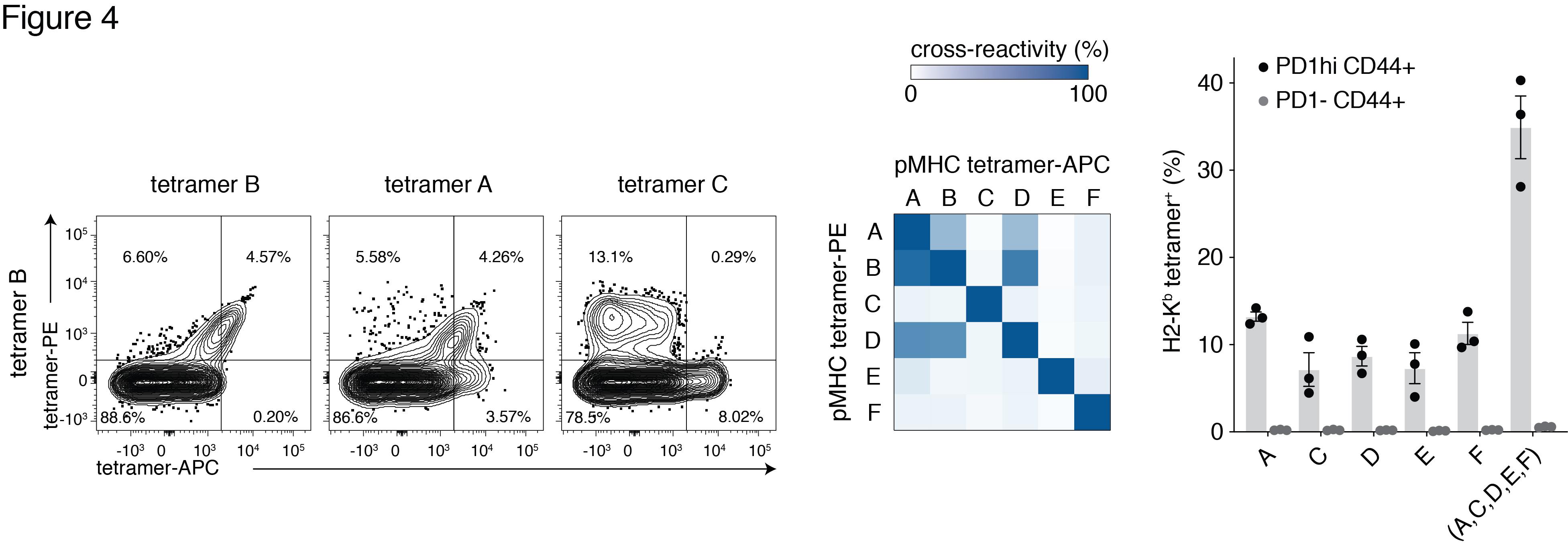
Six pMHC epitopes were selected to survey the recognition of multiple pMHC specificities by alloreactive T cells. Cross-reactivity profiles of these 6 pMHC epitopes were generated (Fig 4). The extent of cross-reactivity varied widely between epitope pairs. One highly cross-reactive epitope was excluded from combinatorial staining. The remaining 5 pMHC tetramers yielded a cumulative increase in alloreactive T cell detection as predicted (Fig 4).
Conclusion: Direct recognition of epitopes comprising donor MHC-I and self-peptides is required for tolerance induction. Epitopes for directly-alloreactive CD8+ T cells that are conserved across sexes and strains can be identified. A polyclonal population of alloreactive T cells recognize multiple distinct pMHC complexes. pMHC multimer panels incorporating these could be used for the detection and tracking of alloreactive T cells.
Sydney Medical School Foundation. Myee Codrington Medical Research Foundation. NHMRC Ideas Grant [APP1183806].
[1] Cunningham, E.C. et al. Gene therapy for tolerance: high-level expression of donor major histocompatibility complex in the liver overcomes naive and memory alloresponses to skin grafts. Transplantation 95, 70-77 (2013).
[2] Paul-Heng, M. et al. Direct recognition of hepatocyte-expressed MHC class I alloantigens is required for tolerance induction. JCI Insight 3 (2018).