A single-centre, five-year experience with DCD heart transplantation
Sarah Scheuer1,2,3, Hong C. Chew2, Claudio Soto1, Arjun Iyer1,2, Ling Gao2, Mark Hicks4, Jeanette Villaneuva2, Alasdair Watson1, Mark Connellan1, Emily Granger1, Kumud Dhital2, Paul C. Jansz1, Peter S. Macdonald1,2,3.
1Department of Heart and Lung Transplant, St Vincent's Hospital , Sydney, Australia; 2Cardiac Transplant Laboratory, Victor Chang Cardiac Research Institute, Sydney , Australia; 3St Vincent's Clinical School, University of New South Wales, Sydney, Australia; 4Clinical Pharmacology , St Vincent's Hospital , Sydney, Australia
Introduction: In 2014 the world first DCD heart transplant with distant procurement (DPP) was performed in Sydney, Australia. Programs are now established in the UK and Europe, as well as a recently commenced multi-centre trial in the USA, with >150 performed worldwide. In established centres, DCD donors account for >20% of heart transplants with excellent early- and mid-term outcomes. Despite these encouraging results, there is still much to be learnt about DCD heart transplantation, and with increasing numbers of hospitals wishing to start DCD heart programs this paper will describe the protocols, lessons learnt and current outcomes from our experience.
Materials and Methods: Forty-one DCD heart transplants were performed at our institution between July 2014 and February 2020, utilising a DPP and ex-situ perfusion protocol. Male or female DCD donors under the age of 55 years with no known history of cardiac disease were accepted for retrieval. Recipient selection was based on blood group and cross-matching outcomes, heart mass, and clinical urgency. The donor hearts were perfused on the TransMedics OCS device as outlined in Table 1. 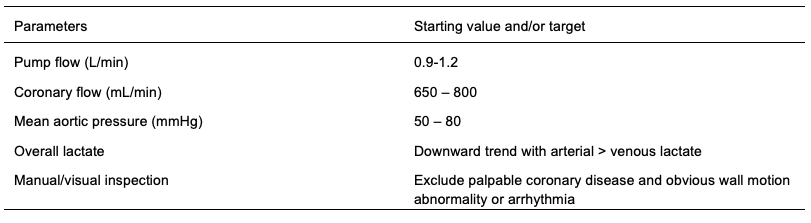 For analysis, donor details were collected using electronic donor records (Donate Life, Australia) with recipient data collected from clinical notes and electronic databases at our institution.
For analysis, donor details were collected using electronic donor records (Donate Life, Australia) with recipient data collected from clinical notes and electronic databases at our institution.
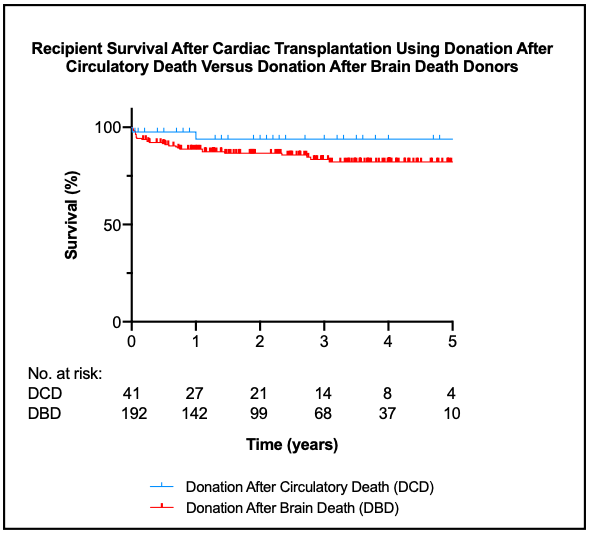
Results: Patients receiving DCD allografts had excellent outcomes with 97.6 and 95.1% 1- and 5-year survival, respectively.
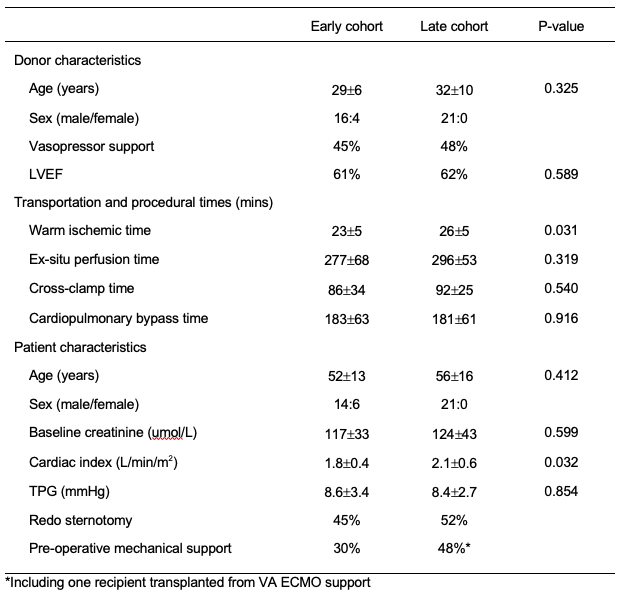
In spite of the similar donor and recipient profiles and a similar rate of utilization of allografts reperfused on the OCS device (74% and 72%, early and late cohort, respectively), the rate of delayed graft function requiring ECMO support has dropped dramatically, from 35% to 14%.
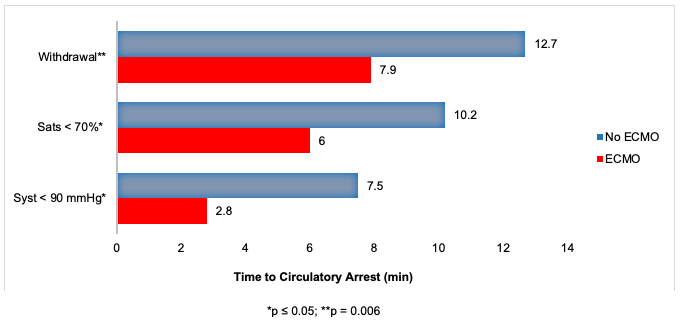
Interestingly, prolongation of the functional warm ischemic time was not associated with an increased rate of ECMO, however prolongation of the asystolic warm ischemic time was. A longer agonal period from withdrawal to circulatory arrest was in fact associated with a lower rate of ECMO.
Discussion: Over our DCD program experience we have made several changes likely to have contributed to the reduced perioperative ECMO requirement. Primarily, we have adopted a more ‘protective’ approach to the management of the heart on the OCS device, targeting in particular lower coronary flows, with a view to reducing endothelial dysfunction and myocardial oedema. In addition, a design change to the OCS module now allows for a controlled cooling of the heart to 16°C prior to delivery of cardioplegia, proving superior protection to the heart during implantation.
Conclusion: DCD heart transplantation clearly provides an additional source of donor allografts with excellent recipient outcomes. Our experiences have reinforced the benefit of a ‘protective’ perfusion strategy on the OCS device, and also raise questions about the true tolerable warm ischemic time, which requires further investigation.
Curran Foundation.
[1] Chew, H.C., et al., Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J Am Coll Cardiol, 2019. 73(12): p. 1447-1459.
[2] Messer, S., et al., Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant, 2017. 36(12): p. 1311-1318.