“First-in-human” clinical trial employing adoptive transfer of autologous thymus-derived treg cells (thytreg) to prevent graft rejection in heart-transplanted children
Esther Bernaldo-de-Quiros1, Manuela Camino2, Juan Miguel Gil-Jaurena3, Eugenia Fernández-Santos4, Nuria Gil2, Esther Panadero2, Constancio Medrano2, Rocío López1, Marta Martínez-Bonet1, Diana Hernández-Flórez1, Megan K Levings5, Lory J West6, Marjorie Pion1, Rafael Correa-Rocha1.
1Laboratory of Immune-regulation, Gregorio Marañón Health Research Institute (IISGM), Madrid, Spain; 2Pediatric Cardiology Unit, Gregorio Marañón Hospital, Madrid, Spain; 3Pediatric Cardiac Surgery Unit, Gregorio Marañón Hospital, Madrid, Spain; 4Good Manufacturing Practice (GMP) Facility, Gregorio Marañón Health Research Institute (IISGM), Madrid, Spain; 5Childhood Diseases Research Theme, BC Children's Hospital, Vancouver, BC, Canada; 6Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada
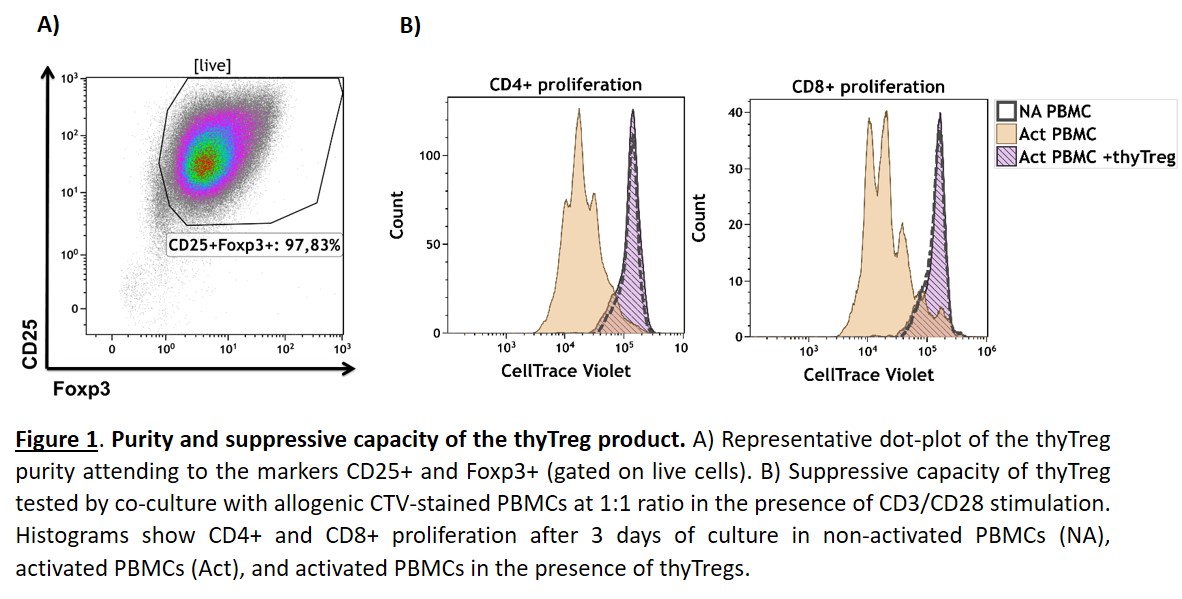
Introduction: Immune allograft rejection remains the main obstacle to successful transplants. Due to their suppressive capacity, adoptive transfer of regulatory T cells (Treg) has acquired growing interest in achieving indefinite graft survival. Limited Treg recovery and reduced quality remain the main obstacles in current protocols where Treg are obtained from adult peripheral blood. We have developed a novel GMP-compatible protocol using the thymus routinely discarded during pediatric cardiac surgeries as Treg source, obtaining massive Treg counts with very high purity and suppressive capacity (Figure 1). Our Treg product has been approved by the Spanish Drug Agency (AEMPS) and is being used to treat transplanted children.
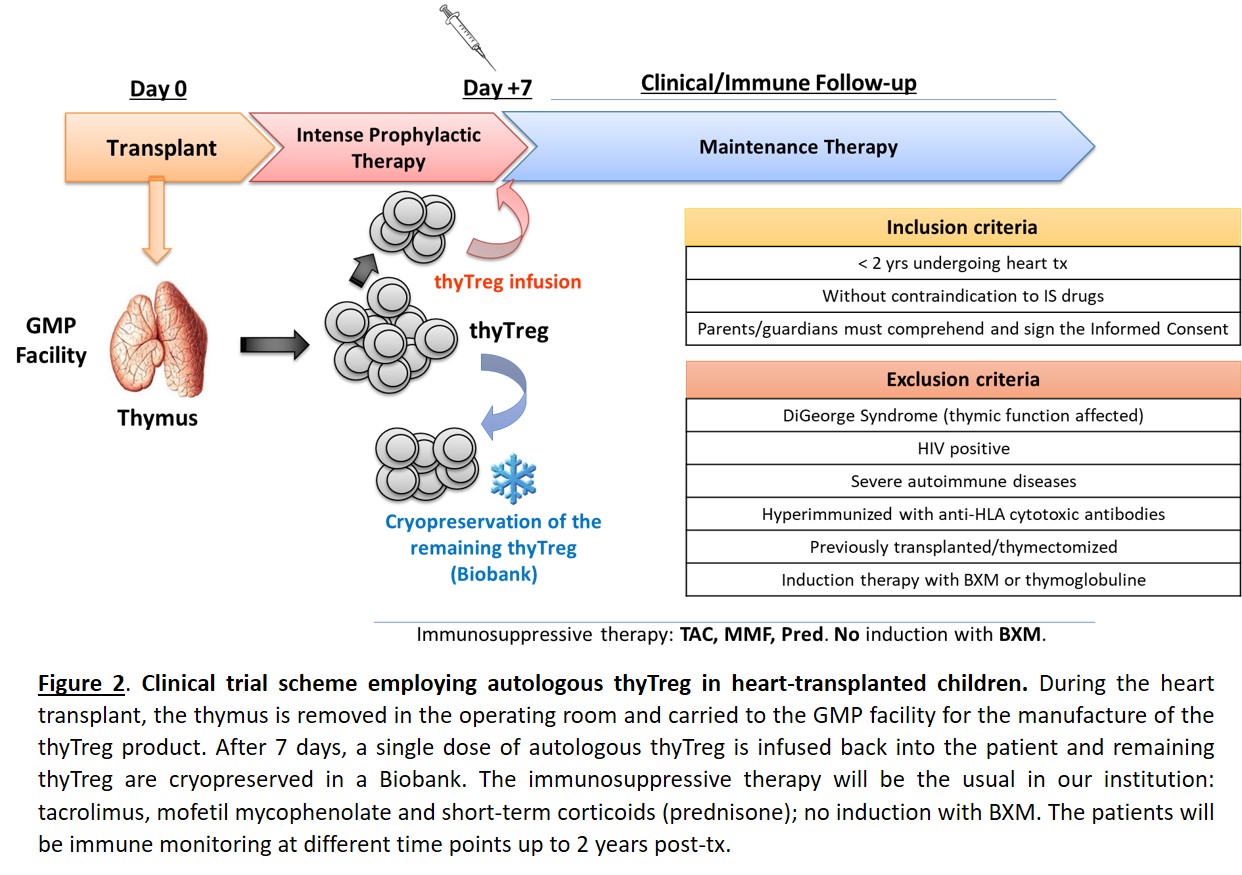
Materials and Methods: We initiated the recruitment of patients last February for the first clinical trial worldwide (phase I/IIa) that evaluates the safety and efficacy of employing autologous thyTreg in 10 children (< 2 years old) undergoing heart transplantation. A single dose of thyTreg, manufactured under GMP conditions, is infused back into the patient at day +7 post-transplant and the remaining cells are cryopreserved for potential future reinfusions. The children are receiving as immunosuppressive therapy: tacrolimus, mofetil mycophenolate and short-term corticoids; without induction therapy with basiliximab (Figure 2). As a control cohort, we have performed a prospective study of 7 heart-transplanted children that were also thymectomiced and receive the same immunosuppressive therapy but not the thyTreg therapy, performing a comprehensive immune analysis at different time points before and up to 2 years post-transplant.
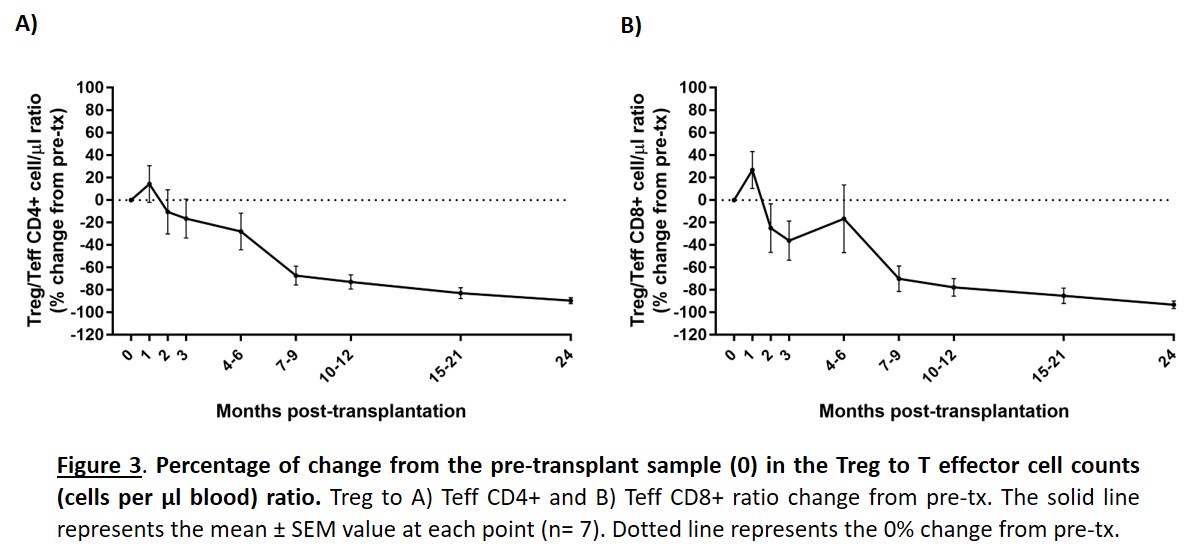
Results and Discussion: The prospective study of our control cohort showed that the Treg to T effector CD4+ and CD8+ ratio significantly decreased after transplant until reaching values of -89,47% and -93,15% lower, respectively, at 2 years post-transplant (Figure 3). The marked decrease in the Treg/Teff ratio reveals that tolerance mechanisms are compromised in heart-transplanted children, just in the period with higher incidence of acute rejection. Due to this fact, the main parameters of efficacy in the clinical trial are to evaluate the recovery of circulating Treg, the Treg/Teff ratio, and determine changes in the incidence of acute rejection during the 2-year follow-up. Preliminary results about the evolution of patients treated with thyTreg will be available at the date of the congress.
Conclusion: The clinical use of a cell therapy with thyTreg is a new therapeutic strategy to induce graft tolerance that could establish a new paradigm in the context of solid organ transplantation. The improved quality and amount of thyTreg obtained with this protocol allow to prepare hundreds of doses that could be administered periodically to induce indefinite immunological tolerance and thus achieve the indefinite survival of the transplanted organ.
This work was supported by grants from Fundación Familia Alonso (FFA-19), grants from ISCIII co-founded by FEDER funds (PI18/00495; PI18/00506; DTS18/00038) and EXOHEP-CM Program (B2017/BMD3727)..