The risk of post-KT outcomes by induction choice differ between older and younger KT recipients
JiYoon Ahn1, Sunjae Bae1,2, Nadia M. Chu1,2, Mark Schnitzler3, Gregory P. Hess4, Krista L. Lentine3, Dorry L. Segev1,2, Mara McAdams-DeMarco1,2.
1Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 2Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States; 3Center for Abdominal Transplantation, Saint Louis University School of Medicine, St. Louis, MO, United States; 4Drexel College of Medicine, Drexel University, Philadelphia, PA, United States
Introduction: Among adult kidney transplant (KT) recipients the risk of post-KT outcomes differs by choice of induction immunosuppression. Older recipients experience a compromised immune response; yet, choice of induction is not tailored by recipient age likely due to a lack of evidence about the association between induction and outcomes like length of stay, acute rejection, death-censored graft failure (DCGF), and mortality among older recipients. Therefore, we compared the risk of these post-KT outcomes by induction (rabbit anti-thymocyte globulin [rATG] versus basiliximab) and tested whether these associations differed between older (≥65 years) and younger (<65 years) KT recipients.
Materials and Methods: Using the Scientific Registry of Transplant Recipient data, we identified 46075 KT recipients (age ≥18) in the United States (1/1/2010-12/31/2016) who received maintenance immunosuppression of tacrolimus, mycophenolate mofetil and steroid at the time of discharge. We estimated adjusted odds ratio (OR) of 1-year acute rejection (AR) and hazard ratios (HR) of DCGF and mortality by using logistic and Cox regression. Relative time to discharge was tested with accelerated failure time model. Then, we tested whether the association between induction and post-KT outcomes differed between older and younger recipients (effect measure modification) using a Wald test. Missing covariates were imputed using chained equations. All models were weighted by propensity score to adjust for confounders.
Results: Of the 46075 recipients, 74.9% received rATG and 25.1% received basiliximab. 66.2% of 9949 older recipients and 77.3% of 36125 younger recipients received rATG. rATG was associated with a decreased risk of AR, death, and discharge but not DCGF; however, the associations between induction choice and AR (interaction p=0.031), death (interaction p=0.006), and time to discharge (interaction p=0.012) differed by recipient age. Compared with basiliximab, rATG was more strongly associated with a decreased risk of AR among older (OR=0.71, 95% CI:0.61-0.84) than among younger recipients (OR=0.87, 95% CI:0.80-0.96). However, there was no association between rATG and mortality among older recipients (HR=1.02, 95% CI:0.94-1.12) but among younger recipients (HR=0.87, 95% CI:0.80-0.94). Finally, rATG was only associated with time-to-discharge among younger recipients (HR=0.88, 95% CI:0.81-0.97) but not among older recipients (HR=1.02, 95% CI:0.96-1.08).
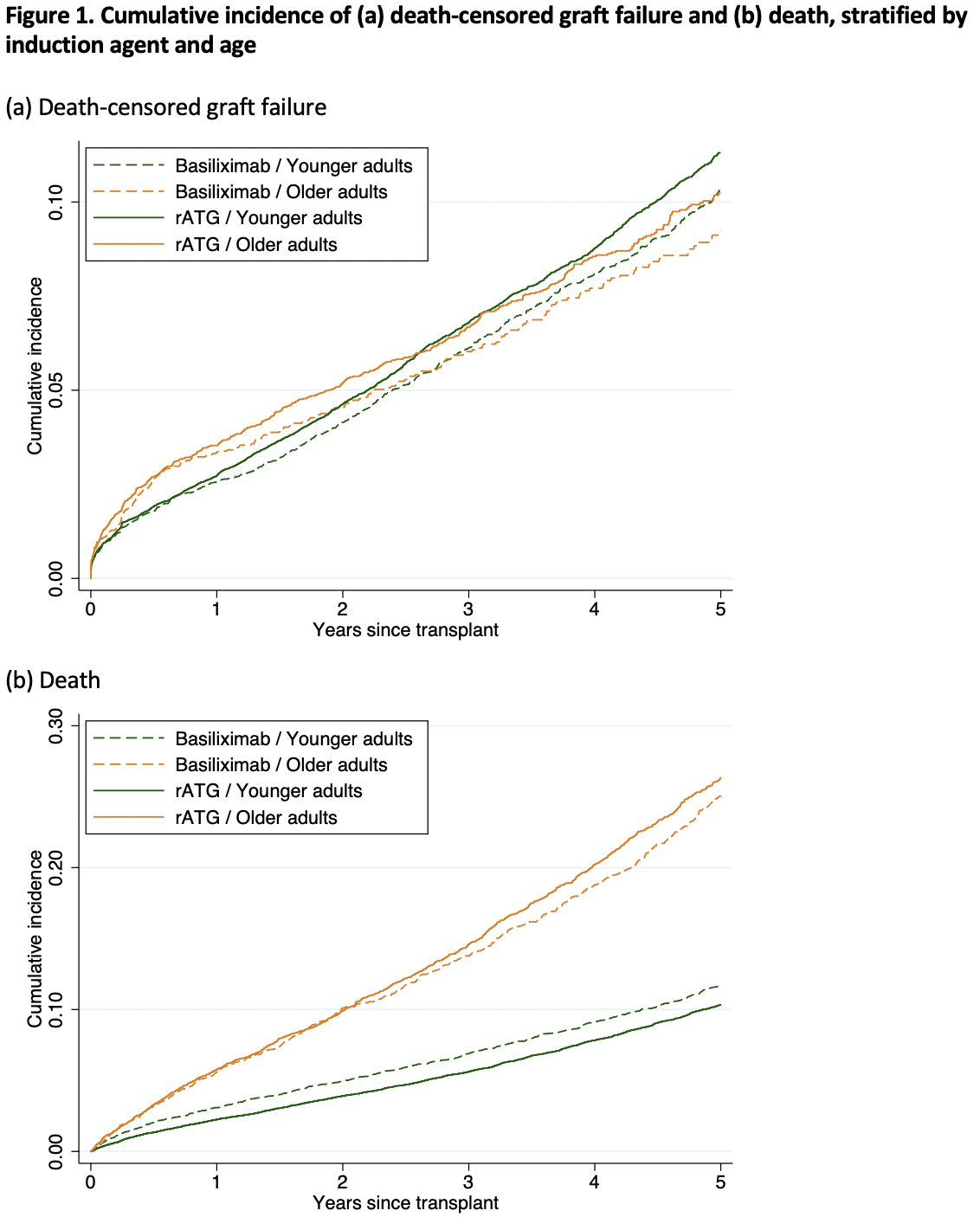
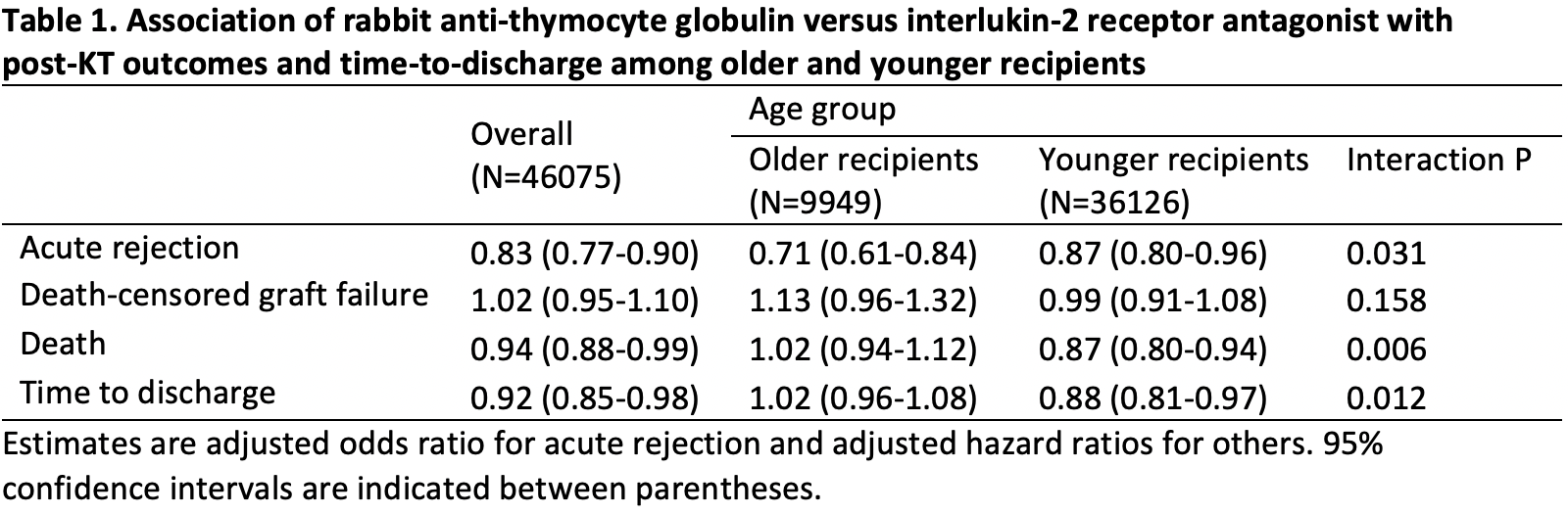
Conclusions: Our findings suggested that rATG may reduce the risk of AR compared to basiliximab more strongly among older KT recipients than among younger KT recipients. Among older recipients, there was no significant evidence of the association between rATG and mortality or length of stay compared with basiliximab. Choice of induction immunosuppression should be tailored by recipient age to reduce the risk of AR in older recipients.
The National Institute of Diabetes and Digestive and Kidney Disease: grant numbers K24DK101828 (PI: Segev) and R01DK114074 (PI: McAdams-DeMarco). The National Institute of Aging: grant number R01AG055781 (PI: McAdams-DeMarco). The American Society of Nephrology (PI: Bae).
[1] Meier-Kriesche HU, Ojo A, Hanson J, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885-889.
[2] Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84(3):285-291.
[3] Danovitch G, Savransky E. Challenges in the counseling and management of older kidney transplant candidates. Am J Kidney Dis. 2006;47(4 Suppl 2):S86-97.
[4] Li F, Thomas LE, Li F. Addressing Extreme Propensity Scores via the Overlap Weights. Am J Epidemiol. 2019;188(1):250-257.
[5] van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681-694.
[6] Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. Journal of Statistical Computation and Simulation. 2006;76(12):1049-1064.