Preclinical study for tolerance induction to liver allografts with rapid immunosuppression withdrawal in Cynomolgus monkeys
Hiroshi Sakai1, Erik Berglund1, Sulemon Chaudhry1, Yojiro Kato1, Joshua Weiner1, Paula Alonso-Guallart1, Nathaly Llore1, Karina Bruestle1, Fanny Fredriksson1, Jeffrey Stern1, Dilrukshi Ekanayake-Alper1, Mercedes Martinez2, Genevieve Pierre1, Makenzie Danton1, Sigal Kofman1, Diane Ordanes1, Jay H. Lefkowitch3, Alina Iuga1, Tomoaki Kato4, Megan Sykes1,4,5, Adam Griesemer1,4.
1Columbia Center for Translational Immunology, Department of Medicine, Columbia University, New York, NY, United States; 2Department of Pediatrics, Columbia University, New York, NY, United States; 3Departments of Pathology and Cell Biology, Columbia University, New York, NY, United States; 4Department of Surgery, Columbia University, New York, NY, United States; 5Department of Microbiology and Immunology , Columbia University, New York, NY, United States
Background: Operational tolerance to liver allografts occurs in highly selected patients, permitting immunosuppression withdrawal usually years after transplantation. There is an unmet need for a safe and reliable protocol of tolerance induction that will permit early immunosuppression withdrawal. Herein, we investigated if tolerance to liver allografts can be achieved in cynomolgus macaques using regimens with or without bone marrow transplantation (BMT).
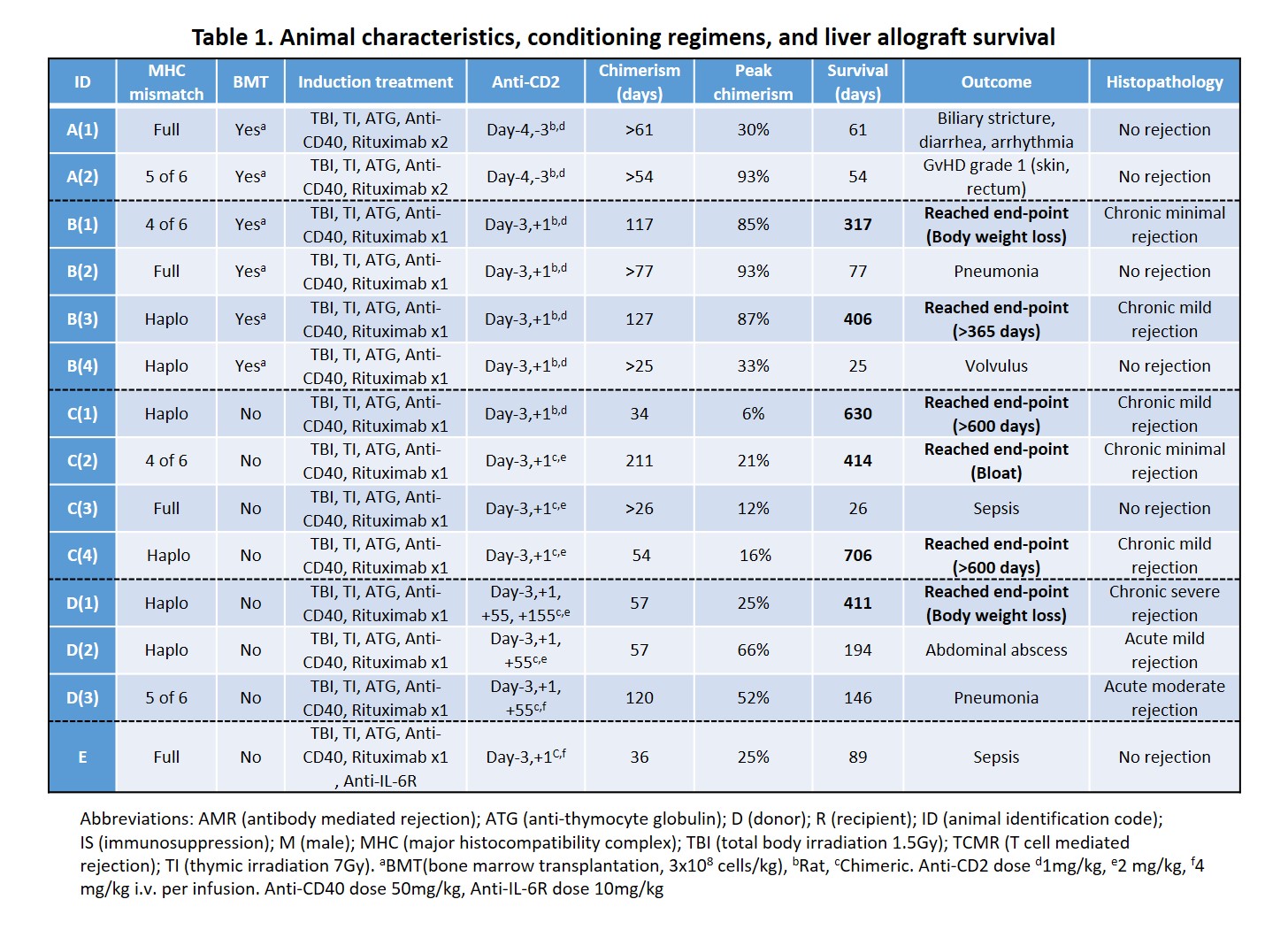
Methods: 14 male cynomolgus monkeys received non-myeloablative immunosuppression regimens in the peri-operative period. Subjects received tacrolimus-based immunosuppression maintenance for 28 days and then complete withdrawal on day 55. We systematically evaluated peripheral blood chimerism, graft function and immunological status until an end-point was reached. Endpoints were graft rejection, graft-versus-host disease (GVHD), weight loss, survival >600 days (> 365 days in Groups A/B) or death. Protocols were modified based on outcomes (Table1).
Results: All recipients developed multilineage mixed chimerism, up to 211days in one subject (Table 1). Animal A(2) developed >80% of donor T-cell chimerism and subsequently had grade 1 GVHD. Addition of anti-CD2 mAb on POD1 in Group B decreased the peak of donor T-cell chimerism and prevented GVHD while permitting myeloid cell chimerism and extending immunosuppression-free graft survival. Recipient CD8+ effector memory T cell (Tmem) numbers recovered, and donor chimerism was lost in association with worsening graft function. In Group C, without BMT, recovery and increase of Tmem were mild even though peak chimerism was lower than in Groups A/B, and long-term graft survival with stable liver function was achieved. Mixed lymphocyte reaction assay showed donor-specific hyporesponsiveness in long-term survivors, while histopathology showed mild chronic rejection. Additional doses of anti-CD2 mAb on POD55 in Group D did not deplete recovered Tmem due to elicited antibodies against anti-CD2 mAb. Increased serum IL-6 levels during a period of bacterial infection correlated with the expansion of Tmem associated with a rejection crisis, while Tmem expansion was absent in Animal E that received anti-IL-6R mAb. PD-L1 expression on antigen-presenting cells was upregulated in the liver allograft of long-term survivors.
Conclusion: This is the first proof-of-concept study demonstrating long-term stable liver allograft function in nonhuman primates using a non-myeloablative treatment regimen with or without BMT to facilitate rapid immunosuppression withdrawal. Controlling the Tmem recovery by agents (e.g. anti-CD2 mAb, anti-CD40 mAb and anti-IL-6R mAb) and the liver’s ability to modulate the activation of host T cells by PD-L1/PD-1 pathway may contribute to tolerance induction to liver allografts.
There are no comments yet...