High-fibre diet reduces transplant-associated dysbiosis and improves renal allograft survival in a murine model of kidney allograft rejection
Julian Singer1,2,3, Huiling Wu1,2,3, Yik Wen Loh1,2, Yan J. Li1,3, Chuanmin Wang1,2, Jian Tan2,4, Laurence Macia2,4, Tony K. Kwan1, Stephen I. Alexander2,5, Steven J. Chadban1,2,3.
1Kidney Node Laboratory, The University of Sydney, Sydney, Australia; 2Sydney Medical School, The University of Sydney, Sydney, Australia; 3Renal Medicine, Royal Prince Alfred Hospital, Sydney, Australia; 4Nutritional Immunometabolism Laboratory, The University of Sydney, Sydney, Australia; 5Centre for Kidney Research, The Children's Hospital Westmead, Sydney, Australia
Aim: To study the effect of kidney transplantation on gut microbial communities, and investigate the role of dietary fibre in modulating host microbiota to improve graft survival in a murine model of kidney transplant rejection.
Methods: Kidney transplants were performed from BALB/c(H2d) to B6(H2b) or B6(H2b):GPR43-/- mice as allografts, and to BALB/c(H2d) as isografts. Allograft mice received normal chow (WT+NC), a HF diet (WT+HF), or sodium acetate (SA) supplementation (WT+SA; GPR43-/-+SA). Isografts were fed NC only. Gut microbiota composition was assessed by 16S rRNA sequencing prior, at post-transplant day 14, and at post-transplant day 100.
Results: In mice fed NC allograft transplantation resulted in a significant shift in gut microbial composition at day 14 post-transplant, which was not evident in isografts (Fig. 1 & 2).
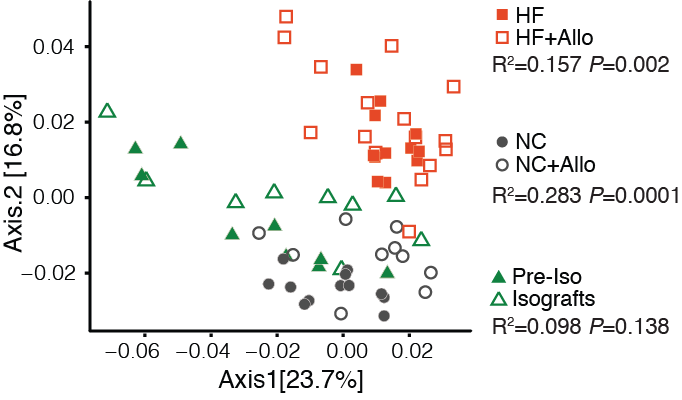
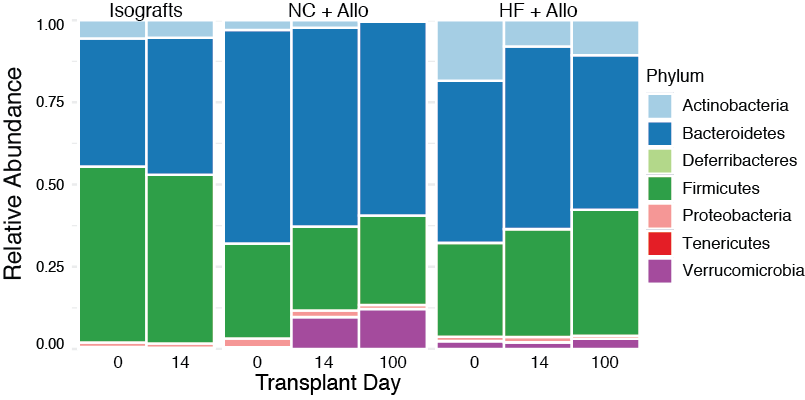
Allograft transplantation led to dysbiosis in WT+NC mice, with a loss of normal gut microbial diversity at day 14, but not in WT+HF mice where microbial diversity was enhanced. Changes in the microbial communities induced by allograft transplantation were maintained at day 100 post-transplant in both NC and HF fed allograft recipients (Fig. 2).
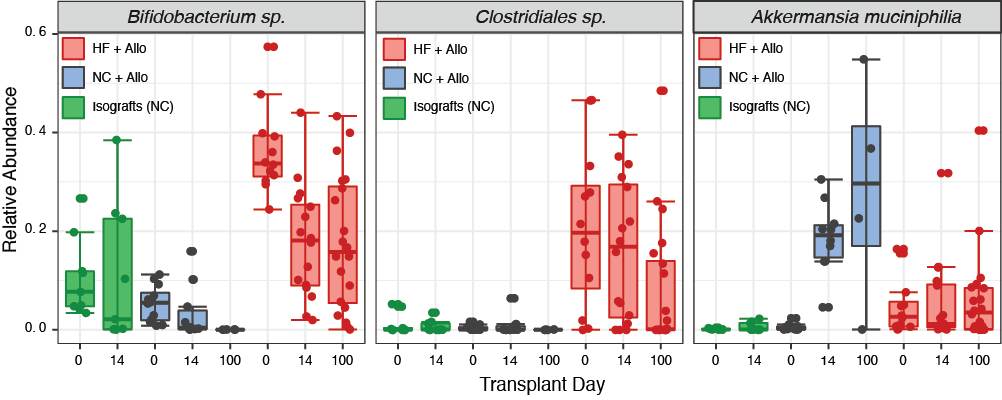
Microbial species known to produce short-chain fatty acid (SCFA) by fermentation of dietary fibre and promote the development of T regulatory cells were significantly more abundant in mice fed HF, compared to NC allograft recipients (Bifidobacterium P<0.05 and Clostridiales P<0.0001, Fig. 3).
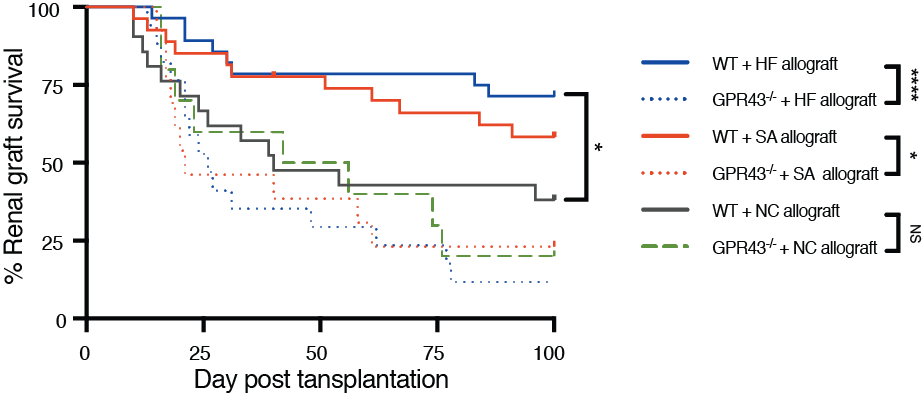
WT+HF allografts had prolonged survival compared to WT+NC allografts (P<0.01, Fig. 4), and were protected from acute (day 14: lower creatinine P<0.01, less tubulitis (P<0.001)) and chronic (day 100: lower creatinine (P<0.05), less proteinuria (P<0.01) and glomerulosclerosis (P<0.001)) rejection.
In mechanistic experiments, WT+SA allografts exhibited superior graft function to WT controls (lower creatinine (P<0.01), were protected from rejection (reduced tubulitis, P<0.001), and exhibited donor-specific tolerance, confirmed by acceptance of donor strain but rejection of 3rd party skin grafts (P<0.01). The survival benefit conferred by SA was broken by depletion of CD25+ Tregs by using anti-CD25mAb, and SA was ineffective in GPR43-/- allograft recipients (P<0.05, Figure 4).
Conclusions: Kidney transplantation resulted in enduring changes to the gut microbial landscape that were not evident in isografts, suggesting the host-donor immune response as the primary driver of transplant-induced dysbiosis. HF diet prevented transplant-associated dysbiosis and afforded protection against allograft rejection. Protection was mediated, at least in part, by SCFAs and was dependent on a CD4+CD25+Foxp3+ regulatory mechanism and signalling via GPR43. Dietary manipulation of the microbiome warrants evaluation in human transplantation.
Supported by NHMRC Project Grant APP1162764.
There are no comments yet...