
Once- versus twice-daily tacrolimus and graft survival
Christian Unterrainer1, Caner Süsal1.
1Institute of Immunology, University of Heidelberg, Heidelberg, Germany
Collaborative Transplant Study.
Introduction: Although the extended-release tacrolimus preparations have the added benefit of a convenient once-daily dosing, large-scale studies evaluating the impact of a possibly improved patient compliance with these preparations are lacking. We analyzed whether the type of tacrolimus at year 1 post-transplant influences subsequent graft survival in adult recipients of deceased donor kidney transplants.
Materials and Methods: There were 12,325 patients who were transplanted since 2011 and were on once- or twice-daily tacrolimus at year 1 post-transplant. An exact 1:1 matching on center, transplant year, graft number, mycophenolate acid and steroids could be performed in 1,250 patients to account for a possible center, transplant year, number of transplant and immunosuppression bias. Univariate Kaplan-Meier analysis was performed before and after exact matching and multivariable Cox regression was applied after matching to adjust for additional confounders.
Results and Discussion: The univariate analysis of graft survival after year 1 in once- and twice-daily tacrolimus patients before and after matching is illustrated in Figure 1.
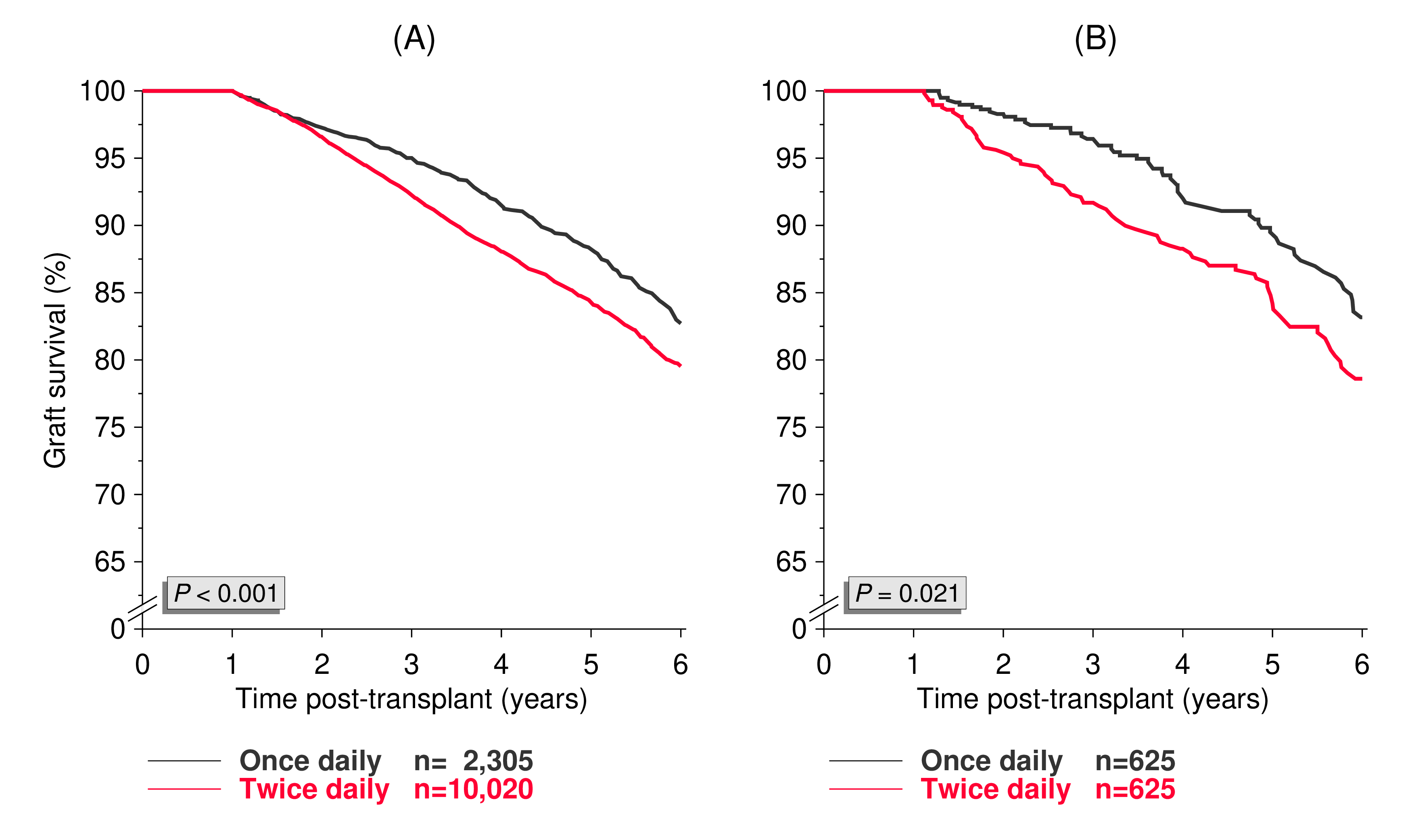
Patients on once-daily formulation had a significantly better outcome (P<0.001 and 0.021, respectively). Figure 2 shows the missing graft survival difference between the two matched groups after year 3.
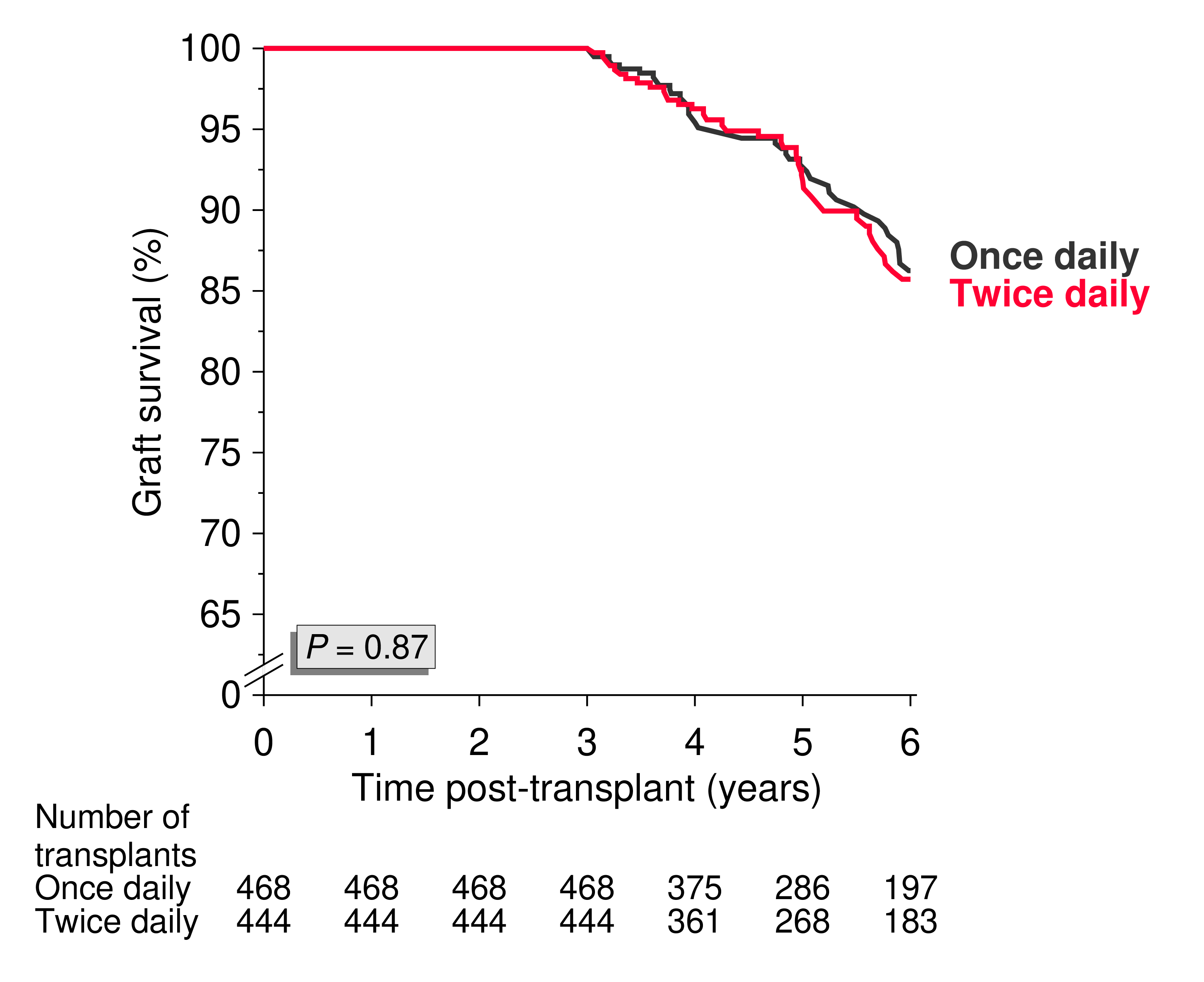
In the multivariable Cox analysis, compared to once-daily tacrolimus patients at year 1, matched twice-daily tacrolimus patients showed a significantly higher risk of death-censored (HR=3.61, P=0.006) and all-cause graft loss (HR=2.31, P=0.003) and a non-significantly higher risk of patient death (HR=1.80, P=0.076) during years 2 to 3. However, in line with the results of the univariate analysis shown in Figure 2, after year 3, the increased risk declined to a nonsignificant difference (HR=1.20, P=0.62; HR=0.69, P=0.19 for death-censored graft loss and patient death, respectively; HR=0.92, P=0.73 for all-cause graft loss). Retrospective comparison of the effects of different immunosuppressive agents is difficult due to a possible patient selection bias. Likely explanations for the observed short-lasting benefit of once- over twice-daily tacrolimus preparations are: a) the benefit is related to a psychological effect and positive motivation due to a “new” medication resulting in a stronger adherence to medication in once-daily tacrolimus patients for a certain time [1]. Indeed, 75% of once-daily tacrolimus patients at year 1 had started with twice-daily at time of transplantation and less than 10% switched to twice-daily at year 2; b) many of the twice-daily patients switched to once-daily, which could have diminished the difference. In the years 2 to 5 post-transplant, only 28% of the switches were from once to twice daily while 72% were from twice to once.
Conclusion: Once-daily tacrolimus appears to have a short-term beneficial effect, mainly on death-censored graft survival.
[1] Kuypers, D.R., et al., Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation, 2013. 95(2): p. 333-40.
There are no comments yet...
