Clinical study of standard- versus reduced-dose tacrolimus combined with generic mycophenolate mofetil in de novo kidney transplantation: A prospective randomized trial
Jun Bae Bang1, Su Hyung Lee1, Chang-Kwon Oh1, Man Ki Ju2, Hee Chul Yu3.
1Department of Surgery, Ajou University Hospital, Suwon, Korea; 2Department of Surgery, Yonsei University College of Medicine, Seoul, Korea; 3Department of Surgery, Jeonbuk National University College of Medicine, Jeonju, Korea
Background: The lowering of calcineurin inhibitor exposure is possibly considered as the proper strategy to prevent calcineurin inhibitor induced nephrotoxicity in kidney transplant. This clinical study was designed to compare the efficacy and tolerability of reduced-dose tacrolimus with standard-dose mycophenolate mofetil (MMF) versus standard-dose tacrolimus with reduced-dose MMF.
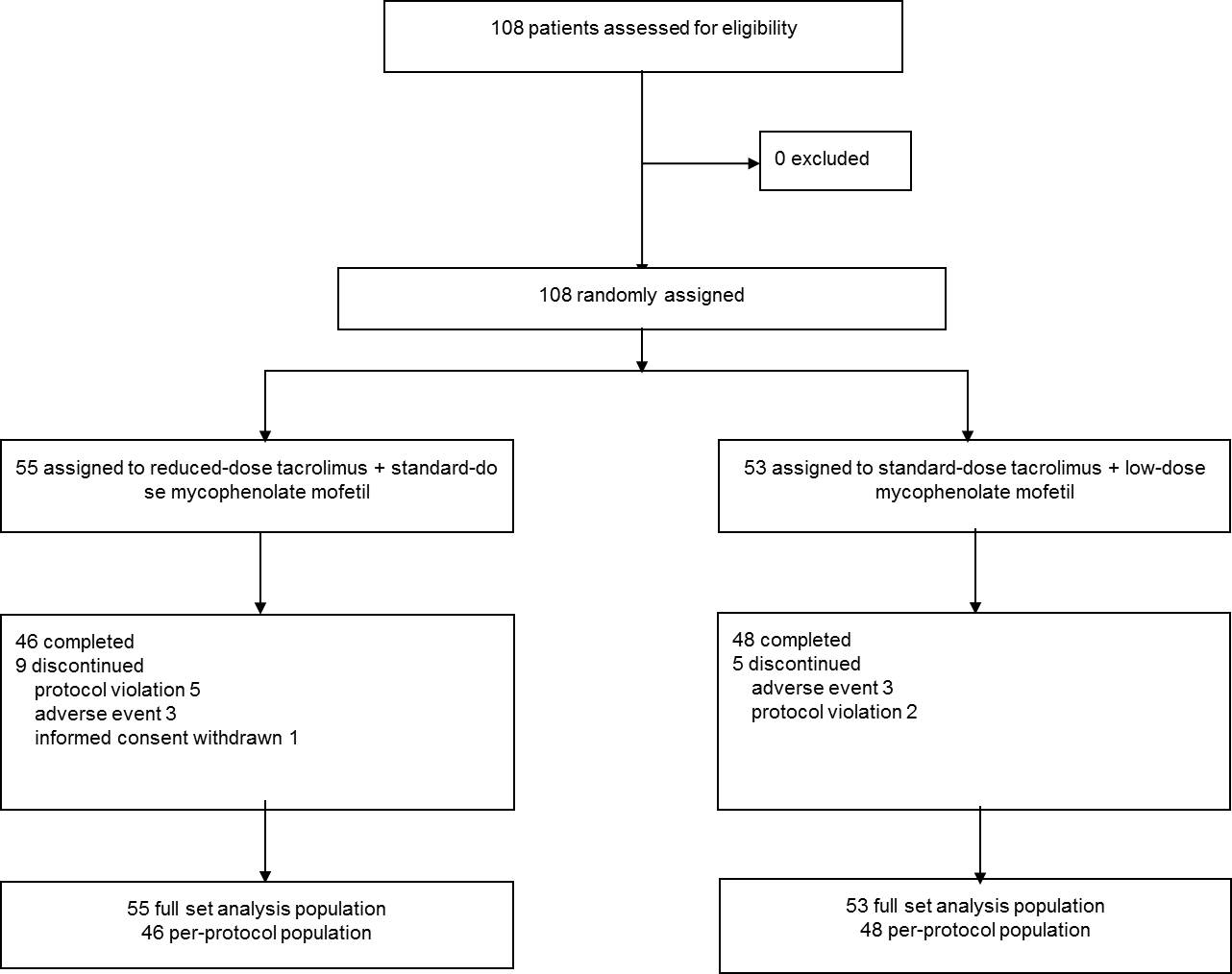
Methods: A prospective, multicenter, open-label, randomized and parallel-group clinical trial was conducted at four transplant centers in Korea. A total sample size was 108 and eligible patients were randomly assigned in a 1:1 ratio to either reduced-dose tacrolimus with standard-dose MMF (the study group) or standard-dose tacrolimus with reduced-dose MMF (the control group) for six months in de novo kidney transplant recipients. Graft function, the incidence of efficacy failure and adverse events were compared.
Results: The mean estimated glomerular filtration rate at six months post-transplantation was 69.83 ± 16.68 mL/min/1.73m2 in the study group and 69.92 ± 17.55 mL/min/1.73m2 in the control group (p > 0.05). The overall incidence of biopsy-proven acute rejection was 3.64% (n = 2) in the study group, compared to 3.77% (n = 2) in the control group (p > 0.05). There was no graft loss, death and loss of follow-up in both groups.
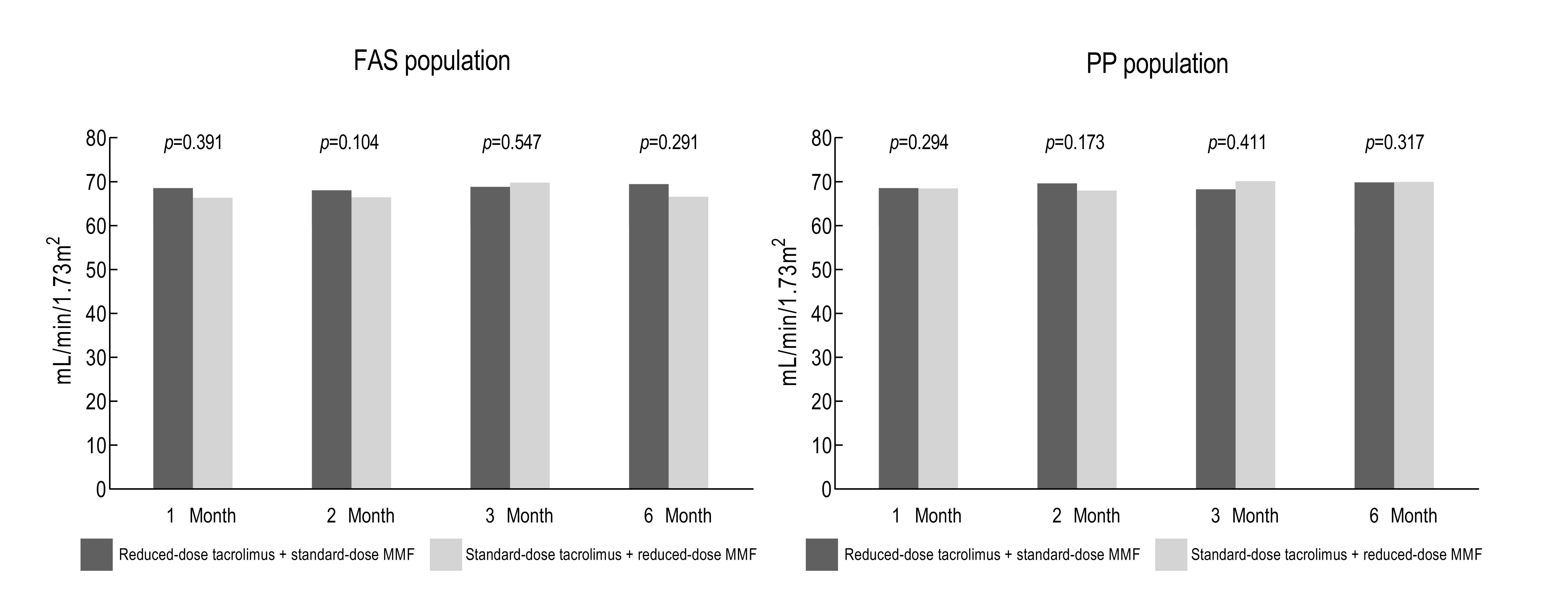
Conclusion: In conclusion, the results suggest that tacrolimus minimization with standard-dose MMF provide adequate immunosuppression with proper renal function and similar rate of incidence of acute rejection compared with the regimen including standard-dose tacrolimus with reduced-dose MMF.
This study was supported by a research grant provided by Chong Kun Dang (Seoul, Korea)..
[1] Meier-Kriesche HU, et el. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378-383
[2] Nankivell BJ, et el. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326-2333
[3] Johnson C, et al. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation. 2000;69:834-841.
[4] Girerd S, et al. Impact of reduced exposure to calcineurin inhibitors on the development of de novo DSA: a cohort of non-immunized first kidney graft recipients between 2007 and 2014. BMC Nephrol. 2018;19:232.
There are no comments yet...
