Genetic modifications attenuate but don’t abrogate the sialoadhesin-dependent adhesion of human RBC to porcine macrophages
Kaitlyn Petitpas1, Ben Cerel1, Zahra Abady1, Margaret Connolly1, Lars Burdorf1, Wenning Qin2, Yinan Kan2, Jacob Layer2, Michele Youd2, William F. Westlin2, Diogo M. Magnani3, Richard N. Pierson III1, Agnes M. Azimzadeh1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2eGenesis Inc, Cambridge, MA, United States; 3MassBiologics of UMMS, Boston, MA, United States
Purpose: Sequestration of human erythrocytes is a prominent observation following liver and lung xenotransplantation. Rees et al previously implicated porcine sialoadhesin in adhesion of human red blood cells (RBC) to wild type (WT) pig macrophages in vitro and ex vivo. The role of genetic modification in pigs lacking carbohydrate antigens, including the pig sialic acid Neu5Gc, has not yet been evaluated in the context of this pathway and overall RBC binding.
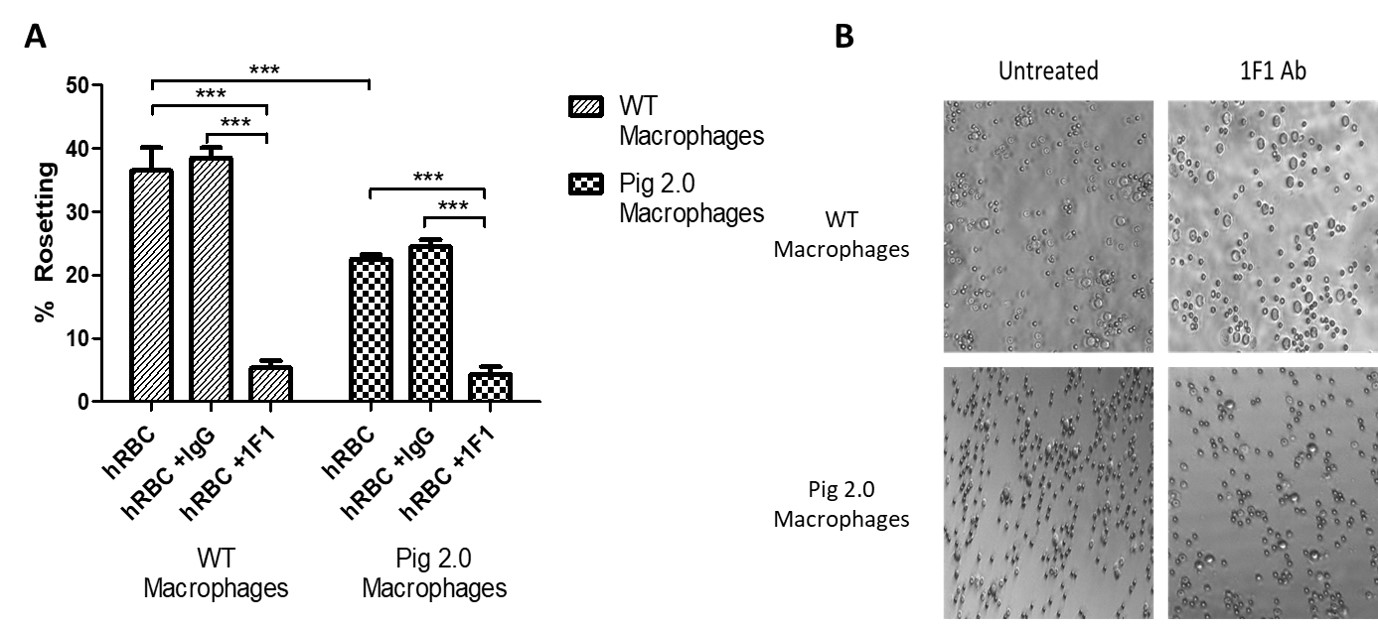
Methods: Porcine alveolar macrophages were isolated from WT (2 different breeds) and Pig 2.0 pigs, which feature Gal1,3αGal, β4Gal and Neu5Gc knockouts and variable expression of human transgenes targeting complement regulation, inflammation, and immune regulation in 3 different lines (Pig 2.05, Pig 2.09, and Pig 2.10). Isolated macrophages cultured in fibronectin-coated 96-well plates overnight were incubated with pig or human red blood cells in the presence and absence of a monoclonal antibody to porcine sialoadhesin (1F1) or isotype control (IgG). Following incubation, wells were imaged (5-10 pictures/well at x10 magnification) to quantify the proportion of macrophages forming rosettes (rosetting was defined as ≥3RBC/macrophage).
Results: WT pig macrophages did not form rosettes with pig RBCs, while rosetting was prominent with human RBCs (36.5±3.7%; n=4). Pig 2.0 macrophages consistently exhibited reduced rosetting (22.5±0.7%; n=8; Figure 1). With the inclusion of the isotype control antibody (IgG), 38.5±1.6% (n=6) of WT macrophages and 24.5±1.1% (n=12) of Pig 2.0 macrophages formed rosettes, displaying no significant differences from the untreated groups. In contrast, rosetting was significantly abrogated (p<0.0001) in both WT (5.3±1.1%, n=6) and Pig 2.0 (4.3±1.17%, n=12) in the presence of 1F1 relative to the isotype control Ab or no treatment. Residual rosetting (~5%) was observed even at high 1F1 concentrations. A Two-Way Analysis of Variance showed significant reductions in rosetting (p<0.0001) both as a result of the genetic modification and the 1F1 treatment.
Conclusion: Genetic modifications attenuate but do not abrogate the adhesion of human RBC to porcine macrophages. Sialoadhesin mediates the majority of binding to both WT and Pigs 2.0 macrophages, confirming its relevance as a therapeutic target. Macrophage rosetting in the presence of 1F1, albeit in small numbers, demonstrates residual binding via an alternate pathway. Current work is exploring a role for Fc-mediated adhesion as well as non-physiologic cross-species interactions between other pig lectins and their human ligand analogues.
There are no comments yet...
