Advanced tertiary lymphoid tissues in protocol biopsies predict progressive graft dysfunction in kidney transplant recipients
Yu Ho Lee1,2, Yuki Sato1,3, Mitsuru Saito4, Shingo Fukuma5, Atsushi Komatsuda6, Nobuhiro Fujiyama7, Shigeru Satoh7, Tomonori Habuchi4, Sang-Ho Lee8, Peter Peter9,10,11, Jürgen Floege10, Motoko Yanagita1,12.
1Department of Nephrology, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 2Division of Nephrology, Department of Internal Medicine, CHA University, Seongnam, Korea; 3Medical Innovation Center TMK Project, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 4Department of Urology, Graduate School of Medicine, Akita University, Akita, Japan; 5Human Health Sciences, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 6Department of Hematology, Nephrology, and Rheumatology, Graduate School of Medicine, Akita University, Akita , Japan; 7Center for Kidney Disease and Transplantation, Akita University, Akita , Japan; 8Division of Nephrology, Department of Internal Medicine, Kyung Hee University, Seoul, Korea; 9Institute of Pathology, RWTH University of Aachen, Aachen, Germany; 10Division of Nephrology, RWTH University of Aachen, Aachen, Germany; 11Electron Microscopy Facility, RWTH University of Aachen, Aachen, Germany; 12Institute for the Advanced Study of Human Biology (ASHBi), Kyoto University, Kyoto , Japan
Background: Tertiary lymphoid tissues (TLTs) are ectopic lymphoid tissues found in chronically inflamed organs. Although studies documented TLT formation in transplanted kidneys, their clinical relevance remains controversial. Here we examined the association of TLTs with future graft function using histological TLT maturity staging we have recently established, the association between TLTs and Banff pathologic scores, and identified the risk factors for developing advanced TLTs.
Methods: Serial protocol biopsy samples (0-hour, 1-, 6-, and 12-month) without rejection were retrospectively analyzed from 181 patients who underwent living donor kidney transplantation. TLTs were defined as lymphocyte aggregates (CD3e and CD20) with signs of proliferation (Ki67), and their stages were determined by the absence (stage I) or presence (stage II) of CD21-positive follicular dendritic cells.
Results: We found multiple TLT-like mononuclear cell infiltrates mainly composed of T cells and B cells.
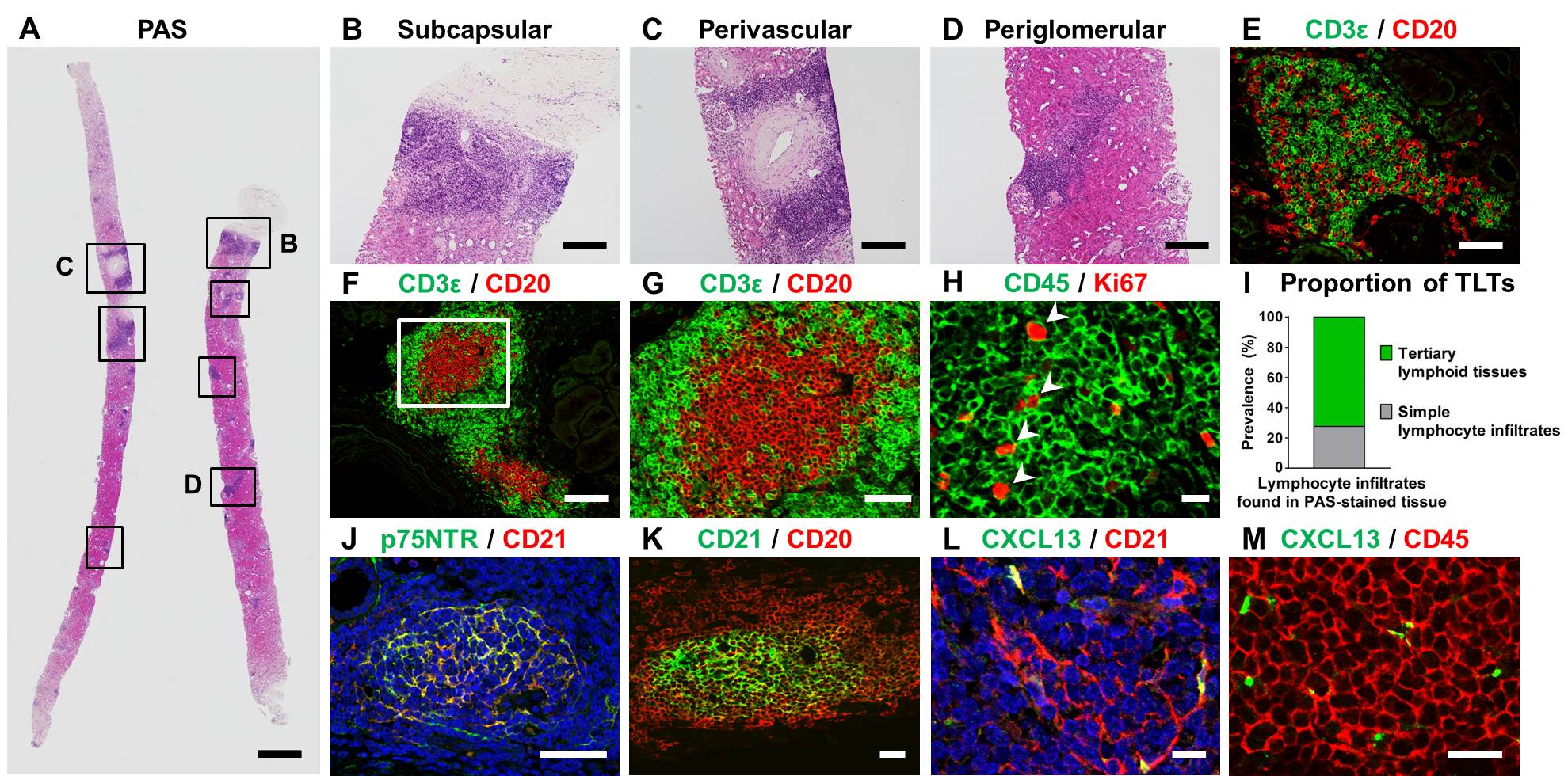
Follicular dendritic cells were also detected in some B cell clusters. While only 5 % of patients exhibited TLTs at 0-hour biopsy, their prevalence increased to almost 50 % at 1-month biopsy and was maintained at similar levels for 12 months. 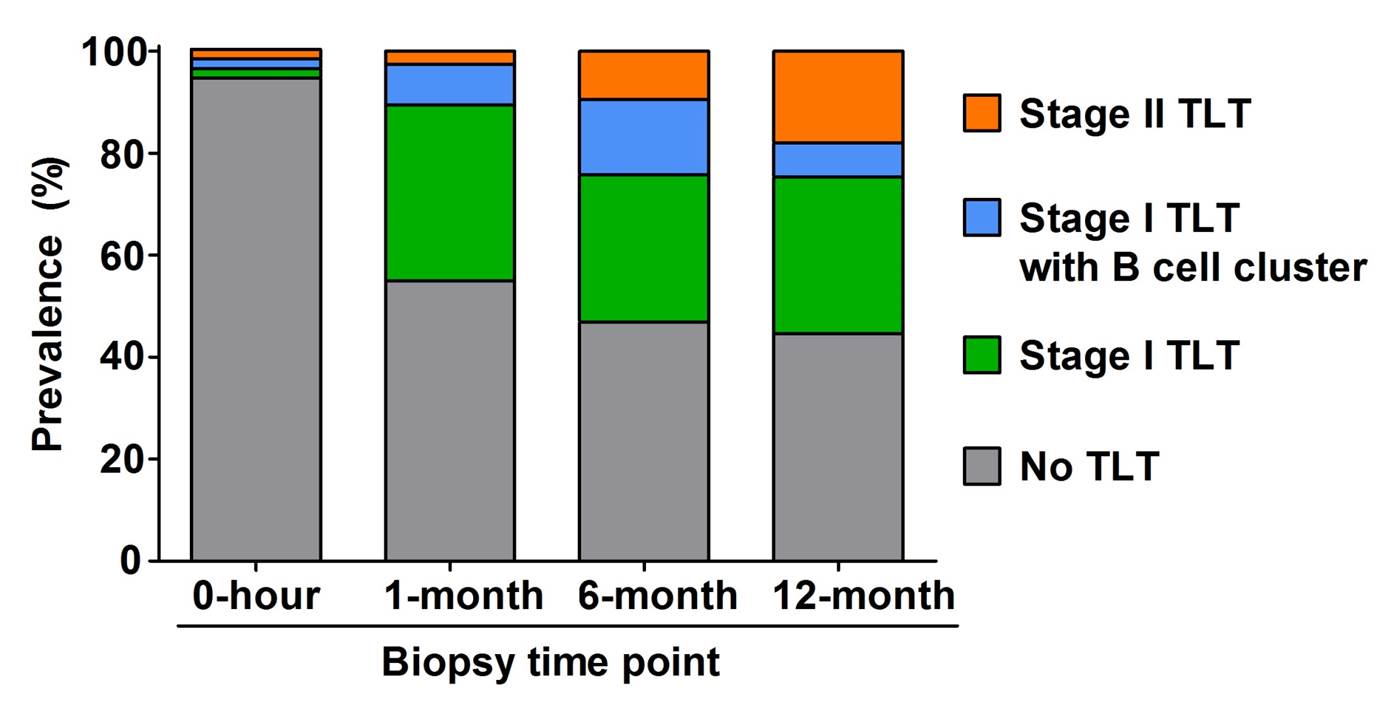
The proportion of advanced stage II TLTs increased gradually, reaching 18 % at 12-month biopsy. Presence of stage II TLTs was significantly associated with lower eGFR over 5 years after transplantation compared to patients with no TLT (adjusted mean difference: -16.33 and -11.88 ml/min/1.73 m2 at 6-month and 12-month biopsies, p = 0.01 and 0.03, respectively).
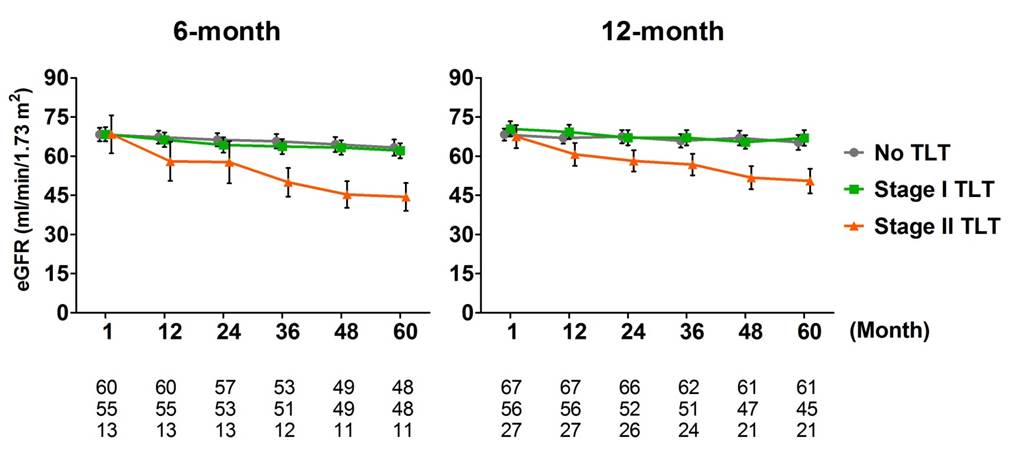
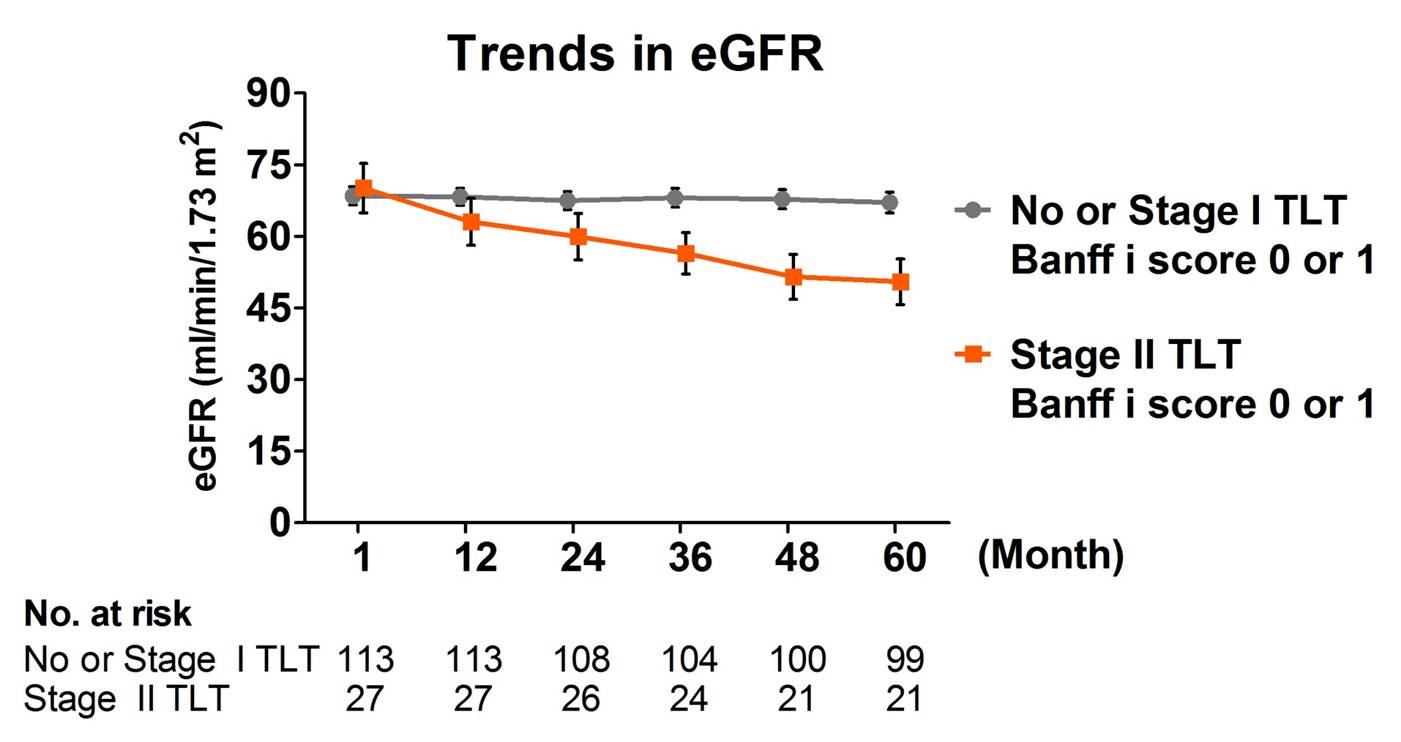
These advanced TLTs were associated with more severe tubulitis and tubular atrophy at 12-month biospsy, and predicted poorer graft function independently from interstitial inflammation (Banff i score).
Risk factor analysis showed rituximab treatment prior to ABO-incompatible transplantation dramatically attenuated the development of stage II TLTs (Odds ratio of 0.18, 95% confidence interval of 0.05 - 0.68, p = 0.01).
Conclusions: TLTs are commonly found in clinically stable transplanted kidneys and advanced stage II TLTs are associated with progressive graft dysfunction independently from interstitial inflammation. Our novel TLT staging strategy has a potential to serve not only as a valuable tool for their systematic classification but also as a predictor of transplant functional decline.
We thank Ms. Kasumoto, Ms. Tomita, Ms. Sakurai, Ms. Nakayama, and Ms. Ozone for their excellent technical assistance. This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 19gm1210009, JP19gm5010002 and JP19gm0610011; partially by grants from the TMK Project, KAKENHI Grant-in-Aids for Scientific Research B (26293202, 17H04187), Grant in Aid for Scientific Research on Innovative Areas “Stem Cell Aging and Disease” from Ministry of Education, Culture, Sports, Science and Technology of Japan (17H05642), Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS), the Translational Research program, Strategic Promotion for practical application of INnovative medical Technology (TR-SPRINT) from AMED; grants from the Uehara Memorial Foundation, Takeda Science Foundation, and the Sumitomo Foundation. This work was also partly supported by World Premier International Research Center Initiative (WPI), MEXT, Japan. This study was co-financed by the German Research Foundation (DFG: SFB/TRR57 and SFB/TRR219, BO3755/3-1 and BO3755/6-1), the German Ministry of Education and Research (BMBF: STOP-FSGS-01GM1518A), and the RWTH Interdisciplinary Centre for Clinical Research (IZKF: O3-7)..
