
Clazakizumab (Anti-IL-6 Monoclonal) treatment of patients with chronic & active antibody-mediated rejection post-kidney transplantation (NCT03380377)
Stanley Jordan1, Noriko Ammerman1, Mieko Toyoda2, Shili Ge2, Edmund Huang1, Alice Peng1, Supreet Sethi1, Reiad Najjar1, Edwin Ortiz2, Maggie Chu2, Abner De Guzman2, Irene Kim1, Kathlyn Lim1, Jua Choi1, Ashley A. Vo1.
1Kidney Transplant, Cedars Sinai Medical Center, Los Angeles, CA, United States; 2Transplant Immunology Laboratory, Cedars Sinai Medical Center, Los Angeles, CA, United States
Introduction: Highly sensitized (HS) patients are at an increased risk for chronic antibody mediated rejection (cABMR) and graft loss due to persistent, deleterious DSA production. Therapeutic options currently available to prevent and treat cABMR are limited. Here, we report our updated, 18 month experience using clazakizumab, a novel anti-IL-6 monoclonal, in HS patients with cABMR + transplant glomerulopathy (TG).
Patients & Methods: Since February 2018, 10 adult patients with biopsy proven cABMR +TG and DSA+ were enrolled in a phase I/II, single-center, open-label exploratory study. All patients received clazakizumab 25 mg subcutaneous injections monthly for 6 doses, underwent a 6-month protocol biopsy, followed by 6 monthly 25 mg doses. After 12 months of therapy, patients were able to enter a long-term extension (LTE) to receive clazakizumab 25 mg subQ every other month. Patients were monitored for DSA relative intensity scores [(RIS); 0 = No DSA; 2 = <5000MFI (weak); 5= 5000-104MFI (moderate);10= >104MFI (strong)], renal function, CRP levels, and T-regulatory (Treg) cell responses.
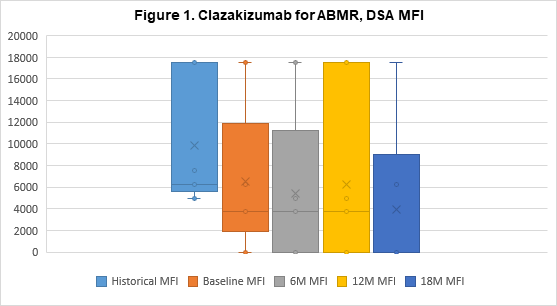
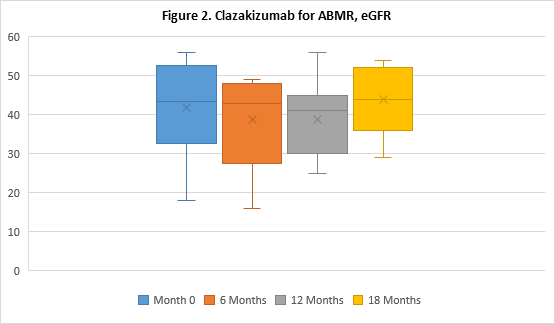
Results: Eight patients continue to undergo clazakizumab therapy, 7 of whom have transitioned to LTE dosing (N=5 have undergone 18M of therapy). Two patients were withdrawn from the study to date, 1 patient progressed to graft failure and withdrew after 4M of therapy, 1 patient preferred to return to Tocilizumab (anti-IL6-R) therapy after 7M. All patients showed marked reductions in CRP levels post-clazakizumab (1.11±1.25 at 0M v 0.43±0.17 at 12M, p=0.31). After 12 months, reductions in mean DSA-RIS were sustained (6.40±3.31 historical vs 3.43±4.58 at 12M, p=0.14) and in 5 patients, DSA-RIS remained reduced at 18M (6.40±3.31 vs 2.50±4.18, p=0.06); see Figure 1 for DSA MFI. 100% of patients’ DSAs were class II (DQ, 80%). Mean eGFRs remained stable at 12M (41.90±12.09ml/min at 0M vs. 38.86±10.42ml/min at 12M, p=0.60); and 18M in 5 patients (41.90±12.09ml/min at 0M vs 44±9.51ml/min at 18M, p=0.74); see Figure 2. Treg cells tended to increase at 12M (2.39±1.02% at 0M vs 3.30±1.13% at 12M, p=0.12). No serious adverse events seen were considered directly related to drug.
Conclusions: cABMR+TG patients treated with clazakizumab showed stabilization of renal function and improvements in DSA RIS after 18 months of therapy. A larger, controlled study is underway to evaluate the potential benefits of clazakizumab in cABMR treatment.
Vitaeris, Inc..
