Carfilzomib and lulizumab-based desensitization prolongs allograft survival in sensitized non-human primates kidney transplantation model
Paul M. Schroder1, Robin Schmitz1, Zachary W. Fitch1, Brian Ezekian1, Janghoon Yoon1, Mingqing Song1, Alton B. Farris2, Ashley Y. Choi1, Andrew Barbas1, Frank Leopardi1, Bradley Collins1, Jean Kwun1, Stuart Knechtle1.
1Surgery, Duke University, Durham, NC, United States; 2Pathology, Emory University, Atlanta, GA, United States
Introduction: Sensitized patients are difficult to transplant due to pre-formed immunity against MHC antigens. We previously reported the benefit of desensitization using carfilzomib and belatacept in a non-human primate (NHP) model. Here we evaluated a desensitization strategy combining carfilzomib (CFZ) with lulizumab (CD28dAb) which, conceptually, has greater immunoregulatory benefit over belatacept by selectively blocking the co-stimulating signal (CD28-B7) whilst preserving the co-inhibitory signal (CTLA4-B7).
Methods: Five maximally MHC-mismatched pairs of NHPs were sensitized to each other with two sequential skin transplants. Individuals from each pair were randomized to receive either desensitization with once weekly treatments of CFZ (27mg/m2 IV) and CD28dAb (12.5mg/kg SC) over four weeks, or no desensitization (Control). This was followed by a life-sustaining kidney transplant between the NHP pairs. All primates received induction therapy with rhesus-specific antithymocyte globulin and maintenance immunosuppression with tacrolimus, mycophenolate, and methylprednisolone.
Results: The desensitized group demonstrated a significant reduction in donor specific antibody (DSA) (ΔMFI -708±83, p<0.05; Fig 1A), Tfh cells (CD4+PD-1+ICOS+; Fig 1B), and proliferating B cells (CD20+Ki67+; Fig1C) in the lymph nodes. Bone marrow plasma cells (CD3-CD14-CD20-CD19+CD38+ or CD3-CD20-CD38+CD138+BLIMP-1+) showed only a marginal reduction. Interestingly, regulatory T cell (CD4+CD25+CD127lo) frequency was maintained after desesnsitization in addition to an increased circulating frequency of naïve CD4 T cells (CCR7+CD45RA+) and naïve B cells (IgD+CD27-CD20+). Treatment with CFZ and CD28dAb significantly prolonged graft survival (MST = 5.8 ± 4.0 vs. 64.8 ± 36.3; p<0.05; FIg 1D) with lower AMR scores (g+ptc; Fig 1E).
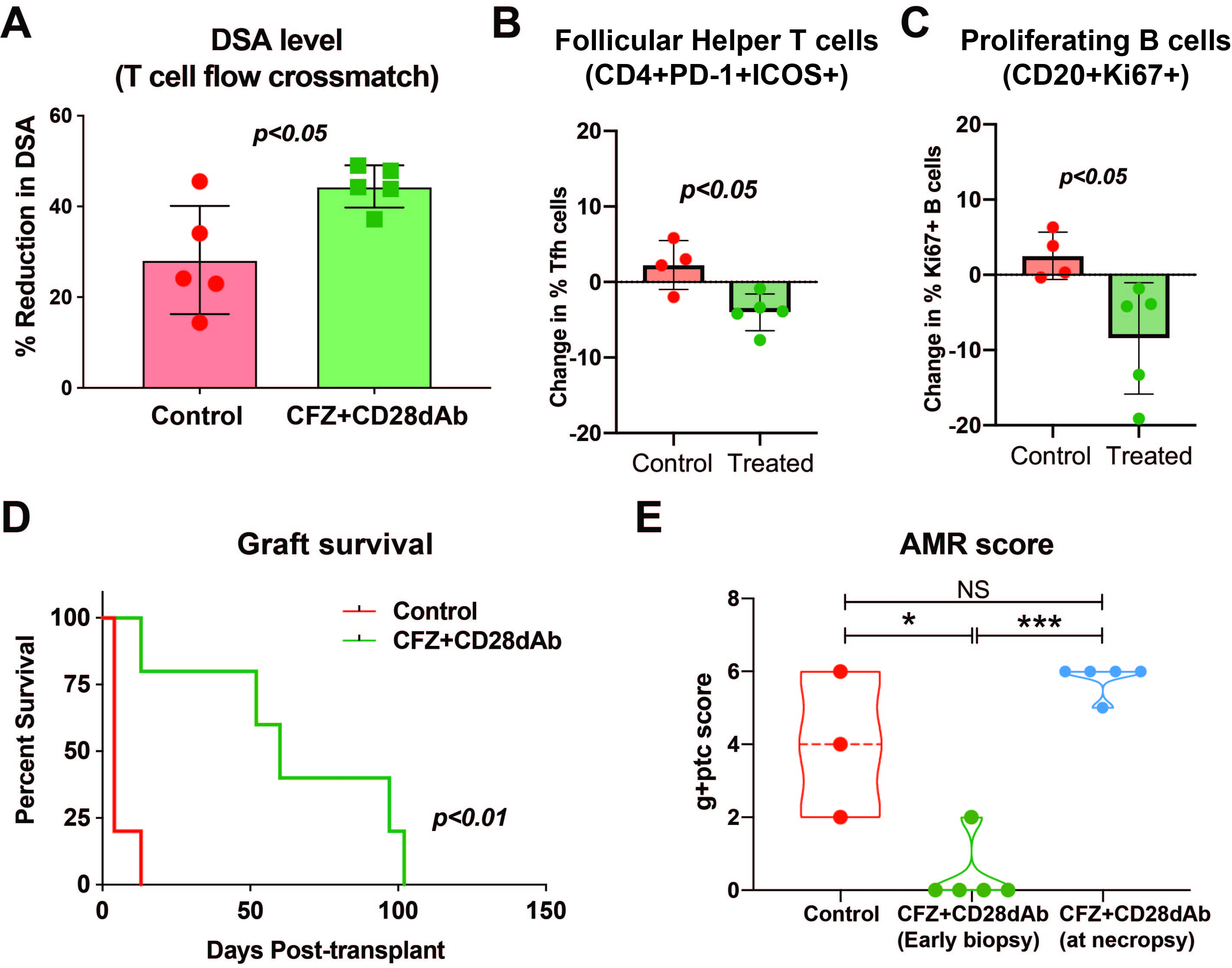
We did not observe clinically relevant CMV reactivation in either group. After kidney transplantation, LN-Tfh cells and proliferating B cells remained reduced in the treatment group. Furthermore, we observed an increase in Tregs. Ultimately, however, all animals showed a rebound of DSA and experienced antibody-mediated rejection.
Conclusions: Desensitization with CFZ and CD28dAb significantly prolongs allograft survival in allosensitized NHPs by reducing DSA and the germinal center response, likely by promoting a more naive lymphocyte repertoire and increasing the frequency of Tregs. Despite this, the strategy failed to promote durable desensitization, suggesting the need for continuous control of the humoral immune response post-transplantation.
