Diagnosis of kidney transplant rejection by tracking donor-reactive T-cell clones in the post-transplant biopsy, blood and urine samples
Joseph R. Leventhal1, Yuvaraj Sambandam1, Jie He1, Xuemei Huang1, Lorenzo Gallon1, James Mathew1.
1Surgery, Northwestern University, Chicago, IL, United States
Introduction: Currently, transplant rejection is monitored by invasive allograft biopsies; hence, a less intrusive method to diagnose rejection is highly desirable. The aim of this study is to test if transplant rejection can be detected by monitoring for donor reactive T-cell clones (DRTCs) in post-transplant biopsies, and non-invasively in blood and urine pellets in renal transplant patients.
Methods: We are conducting a single-center non-randomized study in 80 renal transplant subjects and this is an interim report in the first 30 subjects who have completed the 12 months study period. Pre-transplant (pre-tx) donor-specific MLRs were performed using recipient CFSE-labeled responder PBMC, the CFSE-diluted CD4 and CD8 recipient responder cells were flow-sorted, and the DRTCs were identified using the immunoSEQ® Assay (Adaptive Biotechnologies, Seattle, WA). During the post-transplant (post-tx) period, the presence of the pre-identified DRTCs were monitored again using the immunoSEQ Assay in renal protocol biopsies obtained at months 3 &12 as well as at for-cause, in peripheral blood and urine samples collected at months 3, 6, 12 and at any for-cause event. There were 12 patients in the for-cause group 5 of which had definitive rejection by Banff scoring and 18 in the stable group.
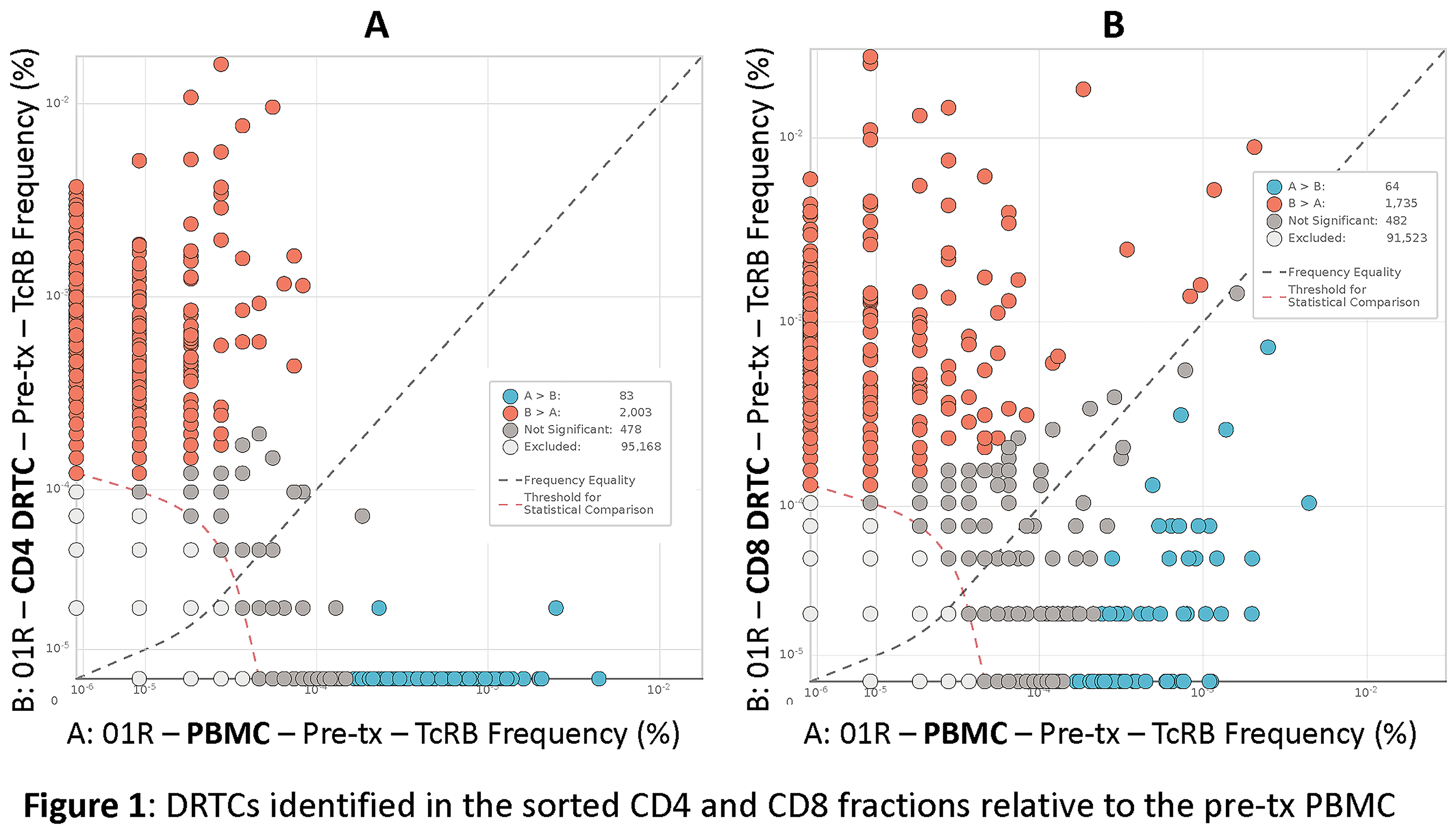
Results and Discussion: Sufficient amount of DNA for the immunoSEQ analysis was obtained from all samples. DRTCs were identified by their enrichment in the sorted CD4 and CD8 fractions of the donor-specific MLR relative to the pre-tx PBMC samples (Figure 1). The pre-tx whole PBMC had both high frequency and rare clones. However, both the high frequency and rare DRTCs were identifiable in post-tx samples.
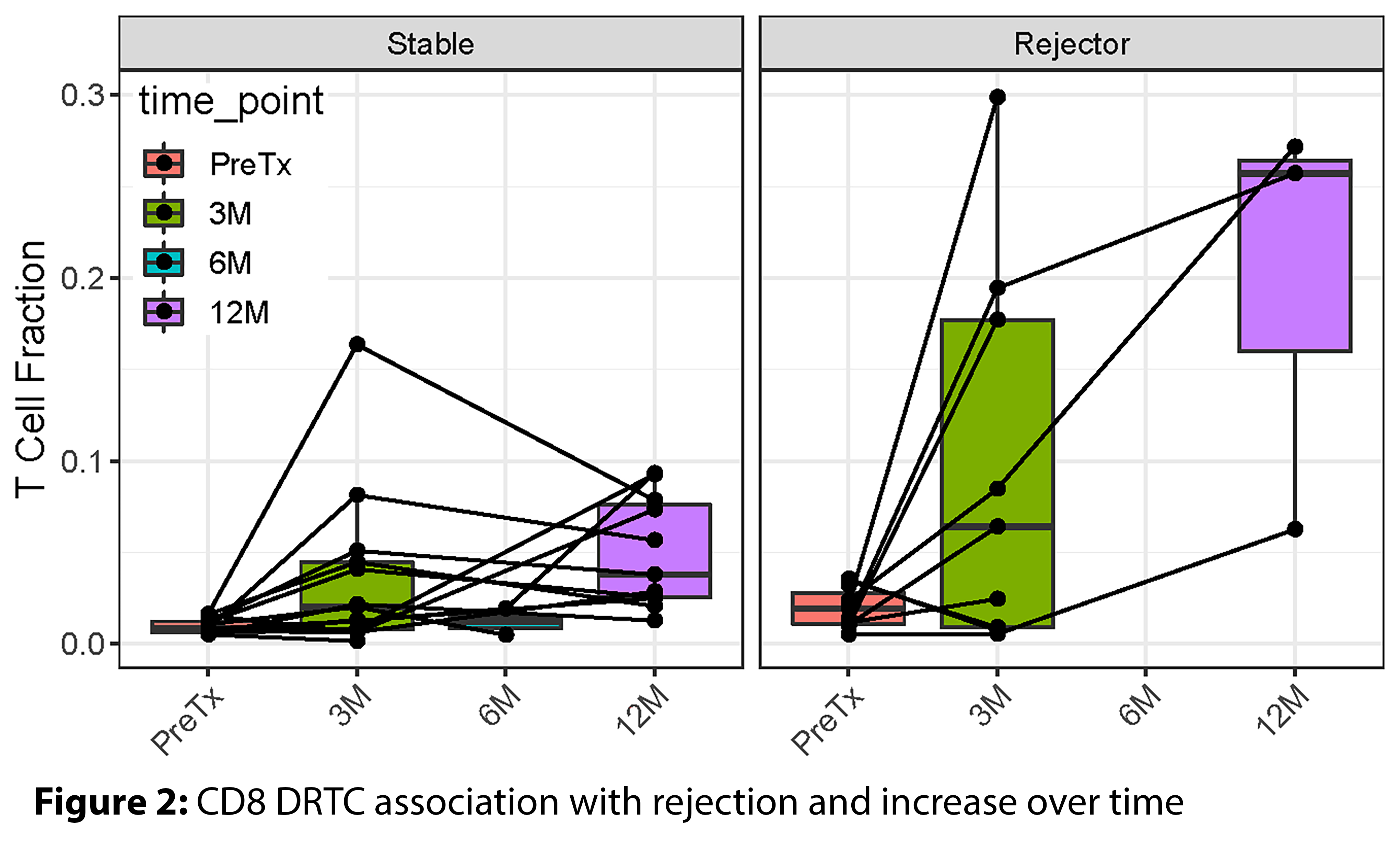
At post-tx, many subjects both for-cause and stable groups had no DRTCs present. If we restrict the analysis to the presence of DRTCs, using a linear mixed model there was a significant association with rejection status (p=0.002) and a significant increase over time (p=0.0003) in renal biopsies (Figure 2). In the peripheral blood, the rejecters vs. the stable group had higher DRTC belonging to the CD8 fraction at 3-month (P=0.02). Similarly, the 6-month urine sample also had a high fraction of T cells identified as CD8 DRTCs in the rejecting group.
Some of the confounding metrics, the significance of which is as yet unknown, included (i) low repertoire clonality and high T-cell fraction in the pre-transplant kidney biopsy was associated with rejection, and (ii) subjects treated with MMF/TAC therapy as opposed to triple immunotherapy had higher peripheral blood T-cell fraction early in the post-tx period (3-months) even though they had the least proportion of rejecting patients.
Conclusion: These preliminary results indicated that our approach at monitoring DRTC shows promise in the ability to diagnosis rejection. Completion of the study also in the remaining 50 subjects may provide a more definitive conclusion.
Department of Defense Grant Funding: W81XWH-17-1-0679.
