Outcomes of non-liver solid organ transplantation using hepatitis C positive donors to hepatitis C negative recipients: A multicenter experience
Kristen Ryland1, Bashar Aqel2, Jorge Mallea4, Shennen Mao5, David Erasmus1, Parag Patel1, Si Pham3, David E. Steidley2, Janna Huskey2, Adyr Moss6, Surakit Pungpapong1.
1Transplantation, Mayo Clinic, Jacksonville, FL, United States; 2Transplantation, Mayo Clinic, Phoenix, AZ, United States; 3Cardiothoracic Surgery, Mayo Clinic, Jacksonville, FL, United States; 4Medicine, Mayo Clinic, Jacksonville, FL, United States; 5Transplant Surgery, Mayo Clinic, Jacksonville, FL, United States; 6Transplant Surgery, Mayo Clinic, Phoenix, AZ, United States
Background: Previously discarded organs infected with hepatitis C virus (HCV) are increasingly being used with the advent of new effective direct-acting antiviral therapy (DAA) therapies for cure, expanding the limited donor pool. We review our experience transplanting kidneys, hearts and lungs from HCV positive donors to HCV negative recipients at 2 high-volume transplant centers.
Methods: This is an ongoing retrospective review of HCV negative kidney and thoracic transplant recipients who received organs from HCV positive donors from May 2019 to present. Descriptive statistics were used to report continuous variables.
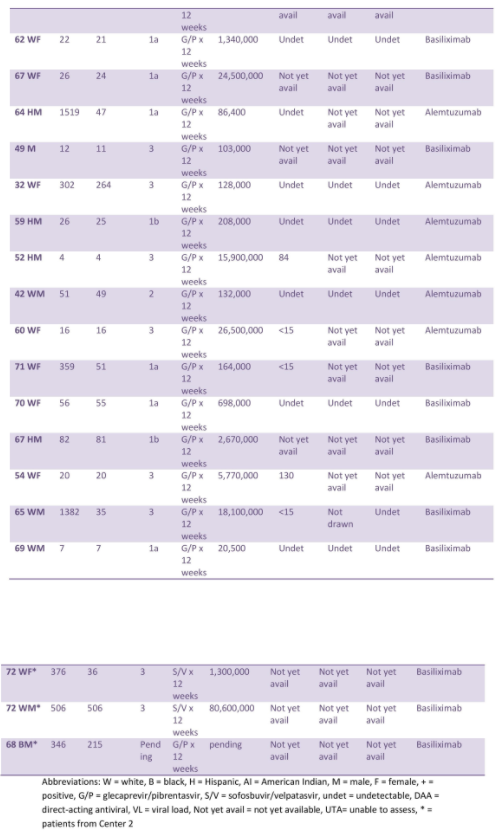
Results: Kidney recipients (n=33):
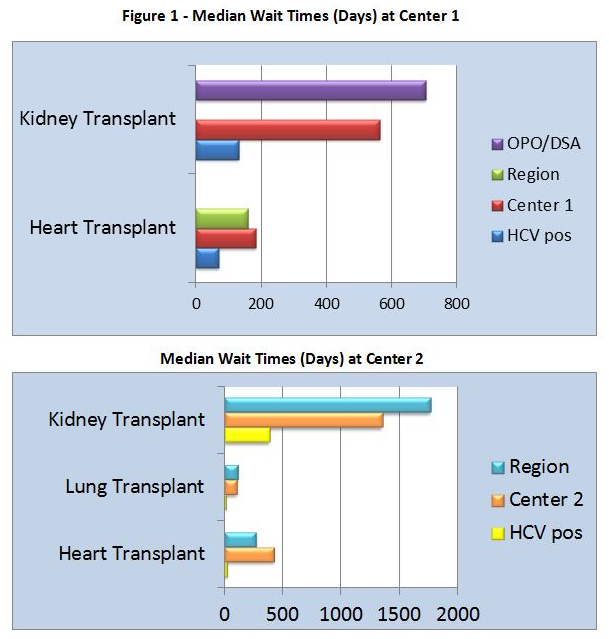
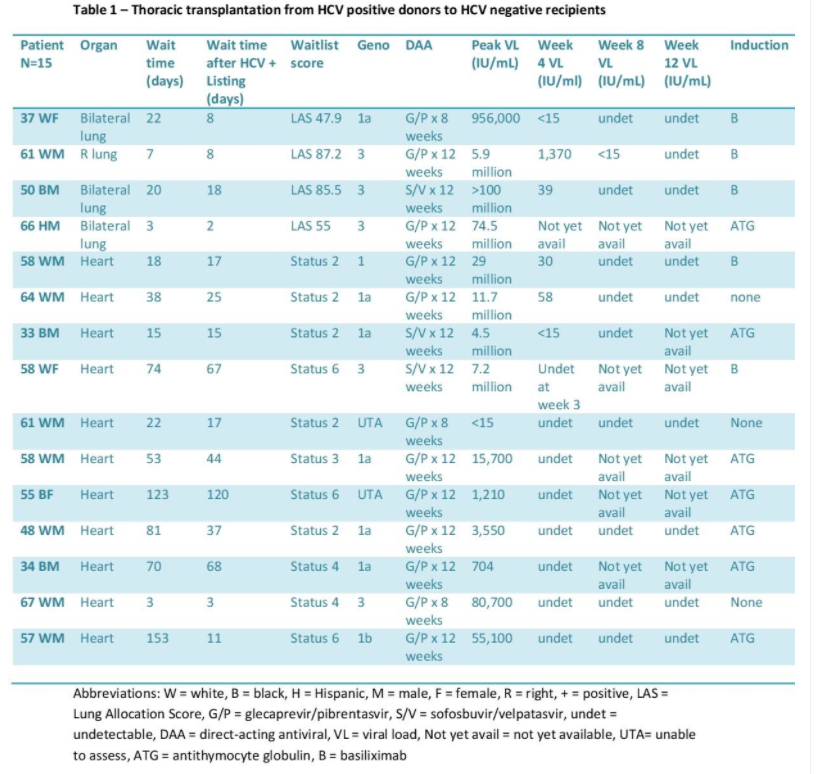
The median age was 64. At Center 1, median time from listing to transplant decreased to 131.5 days, compared with 564 days for standard of care (SOC). At Center 2, median time from listing to transplant decreased to 456 days, compared with 1,323 days for SOC. All patients except 1 developed HCV viremia and required DAA. Median time from transplant to treatment was 20.5 days. Mean peak viral load (VL) prior to DAA was 17,911,706 IU/mL.
While on DAA, HCV RNA at week 4 was 130 IU/mL or less, and all achieved undetected HCV RNA at week 8 and 12.
One patient each had biopsy-proven antibody mediated rejection, possible subclinical rejection versus BK nephropathy, biopsy-confirmed BK nephropathy, and CMV viremia, while three patients developed BK viruria. There was no acute cellular rejection.
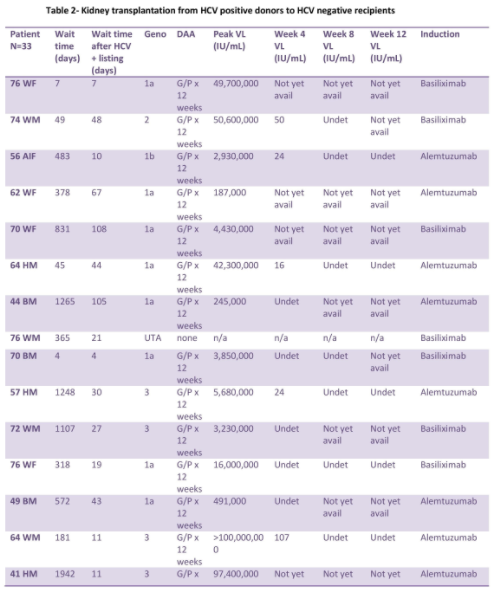
Thoracic recipients (n=4 lung and 11 heart):
The median age was 58. At Center 1, median time from listing to heart transplant decreased to 70 days, compared with 183 days for SOC. All recipients had HCV viremia and received DAA. DAA was started at a median of 4 days post-transplant with the mean peak VL prior to DAA initiation of 22,425 IU/mL. At Center 2, median time from listing to lung transplant decreased to 13.5 days, compared with 108 days for SOC. Median time from listing to heart transplant decreased to 28 days, compared with 429 days for SOC. Median time from transplant to DAA was 35.5 days with the mean peak VL prior to DAA of 29,000,000 IU/mL. Medication regimens were adjusted prior to the start of DAA for drug to drug interactions.
All patients that finished DAA had HCV RNA undetected at week 12. No episodes of acute rejection requiring treatment were noted, with the exception of heart recipients diagnosed with mild grade 1R. There were no adjustments required of immunosuppression regimen directly related to DAA use. There was no CMV reactivation in the thoracic patients. There were no reported adverse events related to DAA use.
Conclusion: Using HCV positive organs greatly shortened the median waitlist time compared with standard of care and did not result in short-term adverse patient or graft outcomes. DAA use reduced viremia and was covered by insurance. A larger multicenter trial to assess the long term effects of HCV positive organs is needed.