
Quantifying the immune response to tissue engineered extracellular matrix
Ryaan EL-Andari1, Sabin J. Bozso2, Michael C. Moon2, Darren H. Freed2, Jayan Nagendran2, Jeevan Nagendran2.
1Medicine, University of Alberta, Edmonton, AB, Canada; 2Cardiac Surgery, University of Alberta, Edmonton, AB, Canada
Purpose: Tissue engineered heart valves have been created in order to reduce the xenoreactive immune response thought to be responsible for structural valve deterioration (SVD) of bioprosthetic heart valve replacements. Our study looks to elucidate whether tissue engineering a commercially available porcine extracellular matrix will significantly attenuate the xenoreactive immune response when compared to the wild-type matrix.
Methods: Samples of whole blood, human pericardium and bone marrow were collected from patients undergoing elective cardiac surgery. We decellularized the wild-type matrix using an established 3 day decellularization process. We then isolated human mesenchymal stem cells (hMSCs) from the collected human bone marrow that we used to recellularize the decellularized matrix. The wild-type, decellularized and recellularized matrix tissues, as well as autologous human pericardium as a control, were exposed to whole blood collected from each patient. On days 1, 3 and 5 samples of blood were collected, centrifuged and the serum stored. Enzyme Linked Immunosorbent Assay (ELISA) was performed to quantify proinflammatory cytokine production.
Results: At days 1, 3 and 5 there was a significant reduction in the concentration of IL-1B cytokine production in each sample of serum exposed to decellularized and recellularized matrix when compared to the wild-type matrix, and a significant reduction in the recellularized matrix when compared to the decellularized tissue. There was a significant reduction in TNF-a concentration at days 1 and 3 for decellularized and days 3 and 5 for recellularized when compared to the wild-type matrix.
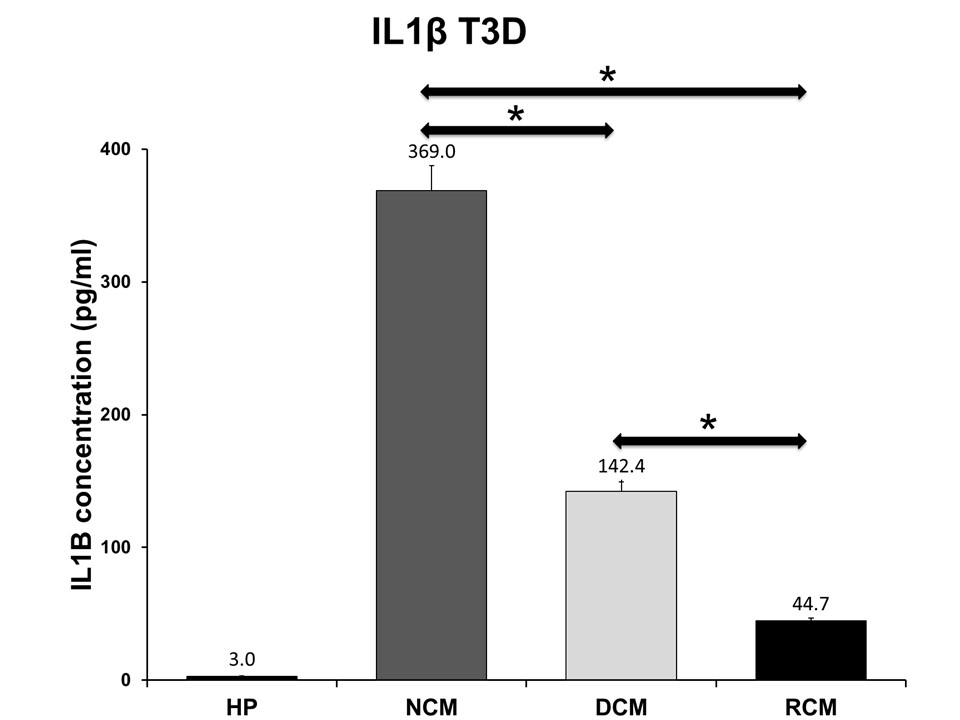
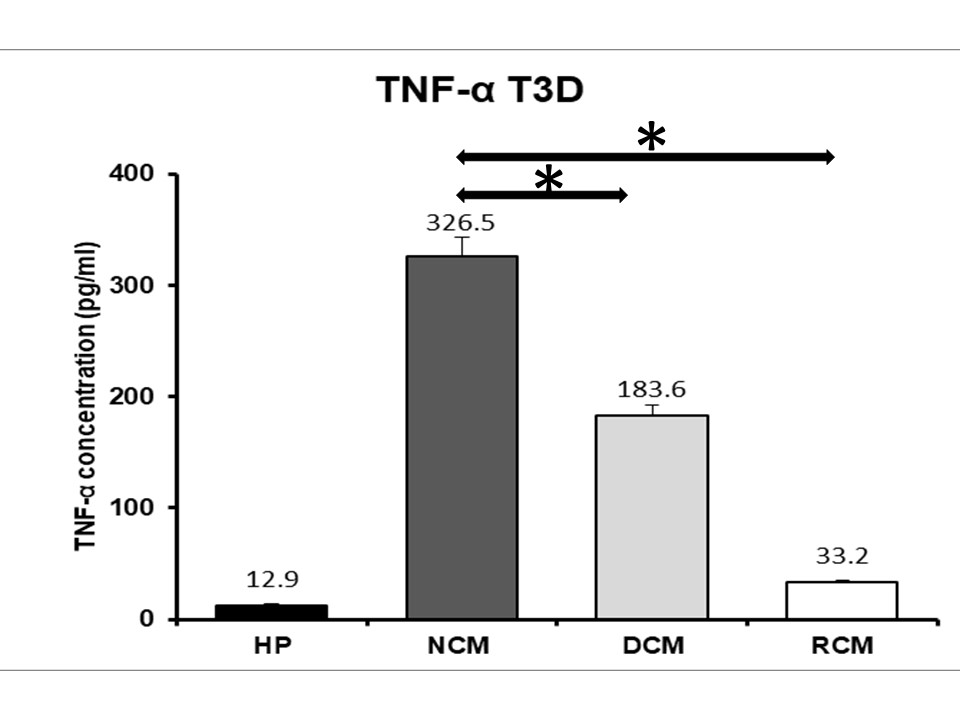
Conclusion: Decllularization and recellularization with autologous hMSCs significantly attenuates the xenoreactive immune response when compared to the wild-type matrix. Therefore, tissue engineering a scaffold for bioprosthetic valve replacements may serve as an effective choice for patients to significantly reduce the xenoreactive immune response previously shown to be responsible for SVD, increasing the durability and longevity of a bioprosthetic heart valve made from this tissue engineered scaffold.
University Hospital Foundation, University of Alberta. Edmonton Civic Employees Research Award.
[1] Lung B and Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014; 30: 962-970
[2] Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG and Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368: 1005-1011
[3] Syedain ZH, Bradee AR, Kren S, Taylor DA and Tranquillo RT. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A. 2013; 19: 759-769
[4] Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008
[5] Jordan JE, Williams JK, Lee SJ, Raghavan D, Atala A, Yoo JJ. Bioengineered self-seeding heart valves. J Thorac Cardiovasc Surg. 2012; 143: 201-208
[6] Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One. 2014; 9: e90406
[7] Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol. 2015; 3: 43
[8] Dignan R, O'Brien M, Hogan P, Thornton A, Fowler K, Byrne D, Stephens F, Harrocks S. Aortic valve allograft structural deterioration is associated with a subset of antibodies to human leukocyte antigens. J Heart Valve Dis. 2003; 12: 382-90; discussion 390-1
[9] O'Keefe KL, Cohle SD, McNamara JE, Hooker RL,Jr. Early catastrophic stentless valve failure secondary to possible immune reaction. Ann Thorac Surg. 2011; 91: 1269-1272
[10] Manji RA, Lee W, Cooper DK. Xenograft bioprosthetic heart valves: Past, present and future. Int J Surg. 2015; 23: 280-284
[11] Seifert M, Bayrak A, Stolk M, Souidi N, Schneider M, Stock UA, Brockbank KG. Xeno-immunogenicity of ice-free cryopreserved porcine leaflets. J Surg Res. 2015; 193: 933-941
