Cyclic helix B peptide ameliorates renal fibrosis induced by unilateral ureter obstruction via inhibiting NLRP3 pathway
Cheng Yang1,3, Ruochen Qi2,3, Weitao Zhang1,3, Ruiming Rong1,3, Tongyu Zhu1,3.
1Urology, Zhongshan Hospital, Fudan University, Shanghai, People's Republic of China; 2Shanghai Medical College, Fudan University, Shanghai, People's Republic of China; 3Shanghai Key Laboratory of Organ Transplantation, Shanghai, People's Republic of China
Background: Renal fibrosis is the inevitable outcome of all progressive chronic kidney diseases and leads to a gradual loss of renal function. We previously reported cyclic helix B peptide (CHBP), a novel synthesized peptide derived from erythropoietin, had shown effective renoprotection. In this study, we investigated the anti-fibrotic effect of CHBP in a murine renal fibrosis model induced by unilateral ureter obstruction (UUO).
Materials and methods: Mice were subjected to the UUO model and CHBP was given intraperitoneally. To assess the therapeutic effects of CHBP, pathological injury, deposition of extracellular matrix and the progression of epithelial-mesenchymal transition (EMT) were examined in vivo. The anti-fibrotic effects of CHBP was validated in vitro using TCMK-1 cells treated with TGF-β1. Involvement of the NLRP3 pathway was demonstrated both in vivo and in vitro.
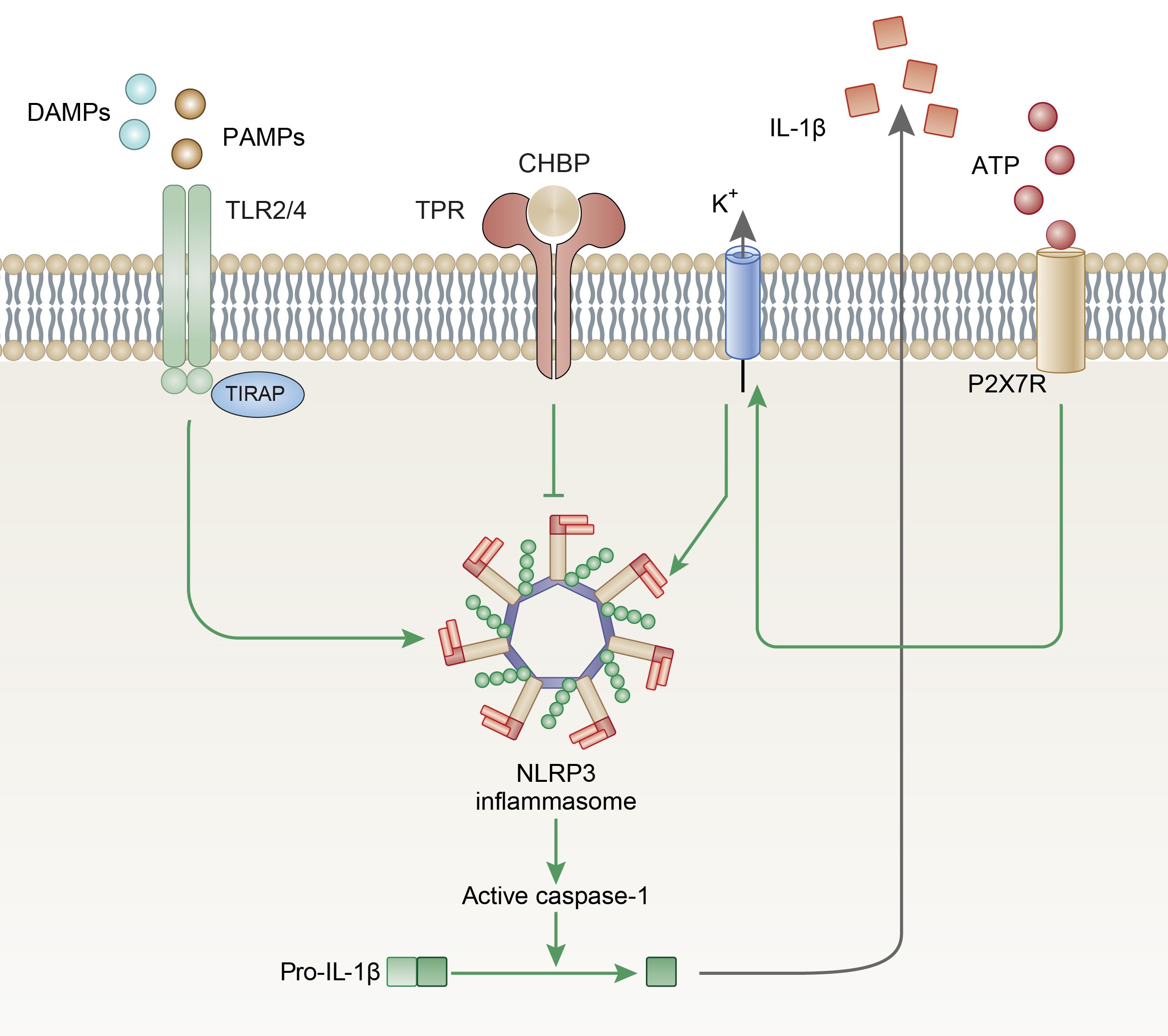
Results: CHBP significantly ameliorated renal tissue injury and fibrosis in terms of extracellular matrix deposition. The EMT process was also alleviated after CHBP treatment. Similar therapeutic effects of CHBP were also observed in vitro in TGF-β1 treated tubular epithelial cells. NLRP3/caspase-1/IL-1β pathway was involved and activated upon injury, both in vivo and in vitro. While the activation of the NLRP3 pathway was found to be in negative correlation with CHBP treatment. CHBP could suppress the activation of NLRP3 and its downstream inflammatory mediators even with addition of extracellular ATP, a direct activator of the NLRP3 inflammasome.
Conclusion: Our results suggest that CHBP could effectively protect the kidney from renal fibrosis in the UUO model via counteracting the NLRP3/caspase-1/IL-1β pathway.
This study was supported by the National Key R&D Program of China (2018YFA0107502 to CY, 2018YFA0107501 to RR), National Natural Science Foundation of China (81770746 to CY, 81770747 to RR), Shanghai Rising-Star Program (19QA1406300 to CY), the Medical and Health Talents Training Plan for the Excellent Youth of Shanghai Municipal (2018YQ50 to CY) and the Science and Technology Commission of Shanghai Municipality (16431902300 to TZ)..
There are no comments yet...
