Primate tolerance model demonstrates longer kidney graft survival with the administration of polyclonal ex-vivo expanded Tregs
Karina Bruestle1, Paula Alonso-Guallart1, Hiroshi Sakai1, Fanny Fredriksson1, Benjamin Piegari1, Emily Lopes1, Genevieve Pierre1, Arthur Shou1, Dilrukshi Ekanayake-Alper2, Joshua Weiner1, Alina Iuga3, Shana M. Coley3, Megan Sykes1, Adam Griesemer1.
1Columbia Center for Translational Immunology, Columbia University, New York City, NY, United States; 2Institute for Comparative Medicine, Columbia University, New York City, NY, United States; 3Department of Pathology and Cell Biology, Columbia University, New York City, NY, United States
Introduction: In humans and non-human primate models, tolerance to donor kidney grafts can be achieved via transient mixed chimerism with concomitant bone marrow transplantation (BMT). We use ex vivo expanded, polyclonal host Tregs at the time of BMT to improve the induction of mixed chimerism. To determine the robustness of tolerance, we waited 120 days before grafting donor kidneys in the abscence of immunosuppression, as such grafts are routinely rejected by animals receiving BMT without Tregs.
Methods: Ex vivo expanded polyclonal recipient Tregs were grown from sorted (CD4+CD25hi) PBMCs and cryopreserved. Conditioning included total body irradiation (125cGy), thymic irradiation (7Gy), anti-CD154 mAbs, horse ATGAM, loCD2 mAbs, followed by allogeneic MHC-mismatched BMT and 1 month of rapamycin, after which all immunosuppression was withdrawn. 8 cynomolgous macaques underwent this regimen, of which 4 received Treg infusions. To test tolerance, donor kidney transplant was performed on day 120 after BMT.
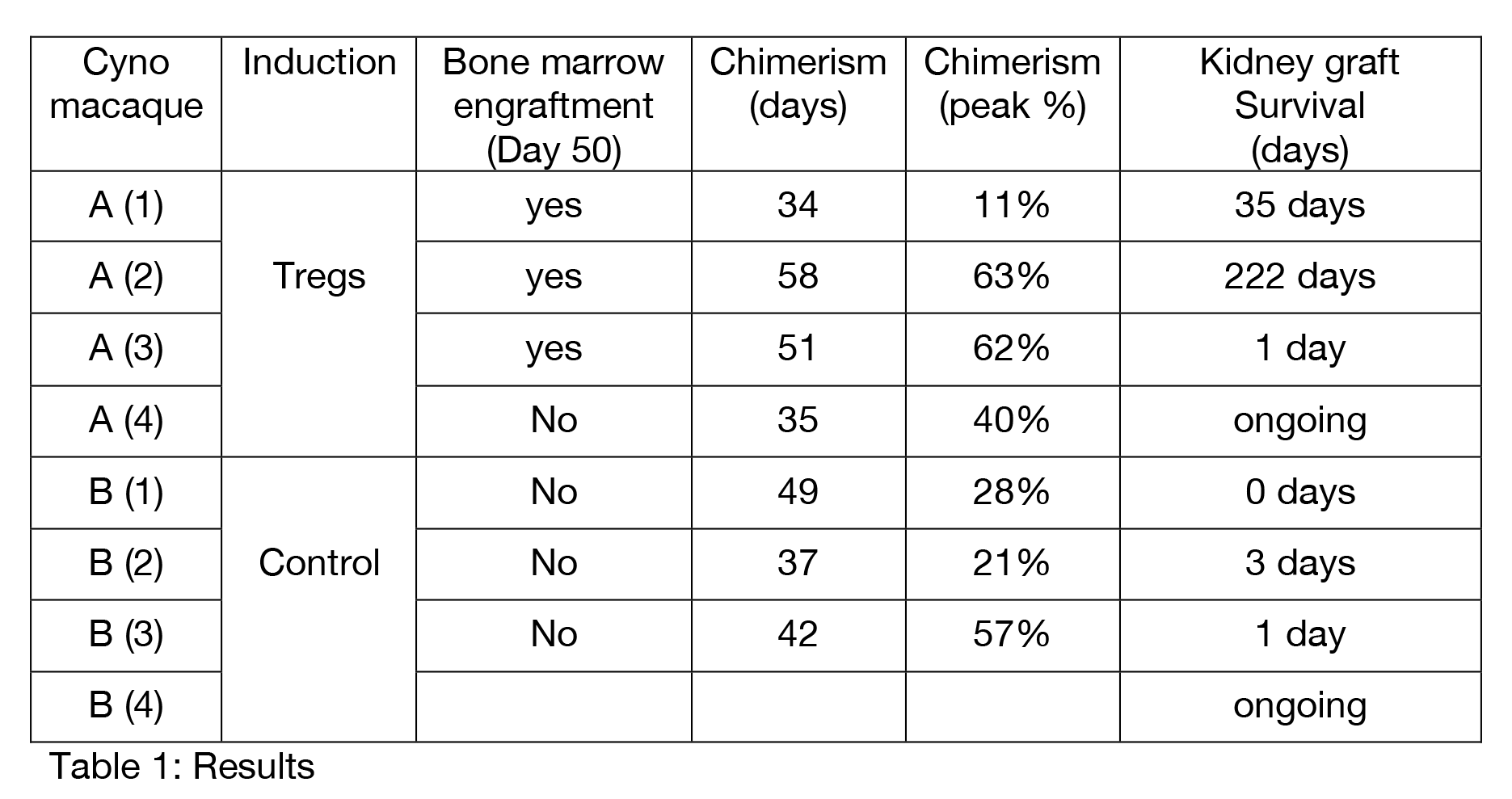
Results: Transient peripheral blood macrochimerism was seen in all animals for 30 to 60 days after BMT. Treg animals showed bone marrow engraftment on day 50, anti-donor hyporesponsiveness in MLR and Elispot, an overall higher level of peak chimerism and no signs of graft-versus-host-disease. In the longest survivor, MLR assays showed anti-donor hyporesponsiveness that was restored in CD25-depleted MLR. This animal also showed a larger Treg population in proliferating anti-donor Tcells versus anti-third party Tcells in a CFSE stained MLR. Treg animals had kidney graft survival up to 222 days without immunosuppresion. In contrast, control animals demonstrated donor-specific anitbodies and rejected their kidney graft within 3 days.
Conclusion: Mixed chimerism in a tolerance model for solid organs can be improved with autologous, ex vivo expanded Tregs. We observed prolonged survival of donor kidneys grafted after peripheral chimerism is lost. Ex vivo expanded, polyclonal Tregs given at the time of BMT not only enhance donor chimerism but may induce a regulatory environment that is associated with prolonged suppression of anti-donor responses.
There are no comments yet...
