Parietal peritoneum as a novel substitute for middle hepatic vein reconstruction during living-donor liver transplantation
Suk Kyun Hong1, Nam-Joon Yi1, Jae-Hyung Cho1, Jeong-Moo Lee1, Kwangpyo Hong1, Eui Soo Han1, Kwang-Woong Lee1, Kyung-Suk Suh1.
1Surgery, Seoul National University College of Medicine, Seoul, Korea
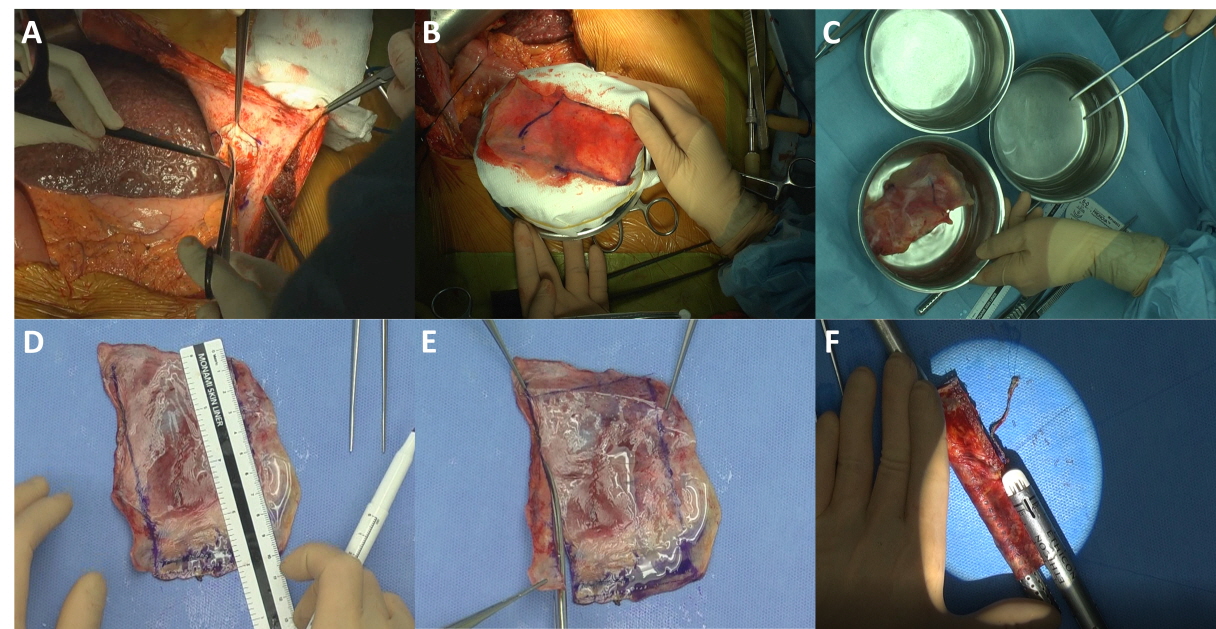
Background: Although autologous, cryopreserved, or artificial vascular grafts can be used as interpositional vascular substitutes for MHV reconstruction during LDLT, they are not always available, are limited in size and length, and are associated with risks of infection. This study aimed to evaluate parietal peritoneum as a novel substitute for middle hepatic vein (MHV) reconstruction during living donor liver transplantation (LDLT).
Methods: Prospectively collected data of 15 patients who underwent LDLT using the right liver with reconstruction of MHV using the patients’ own parietal peritoneum graft were retrospectively reviewed.
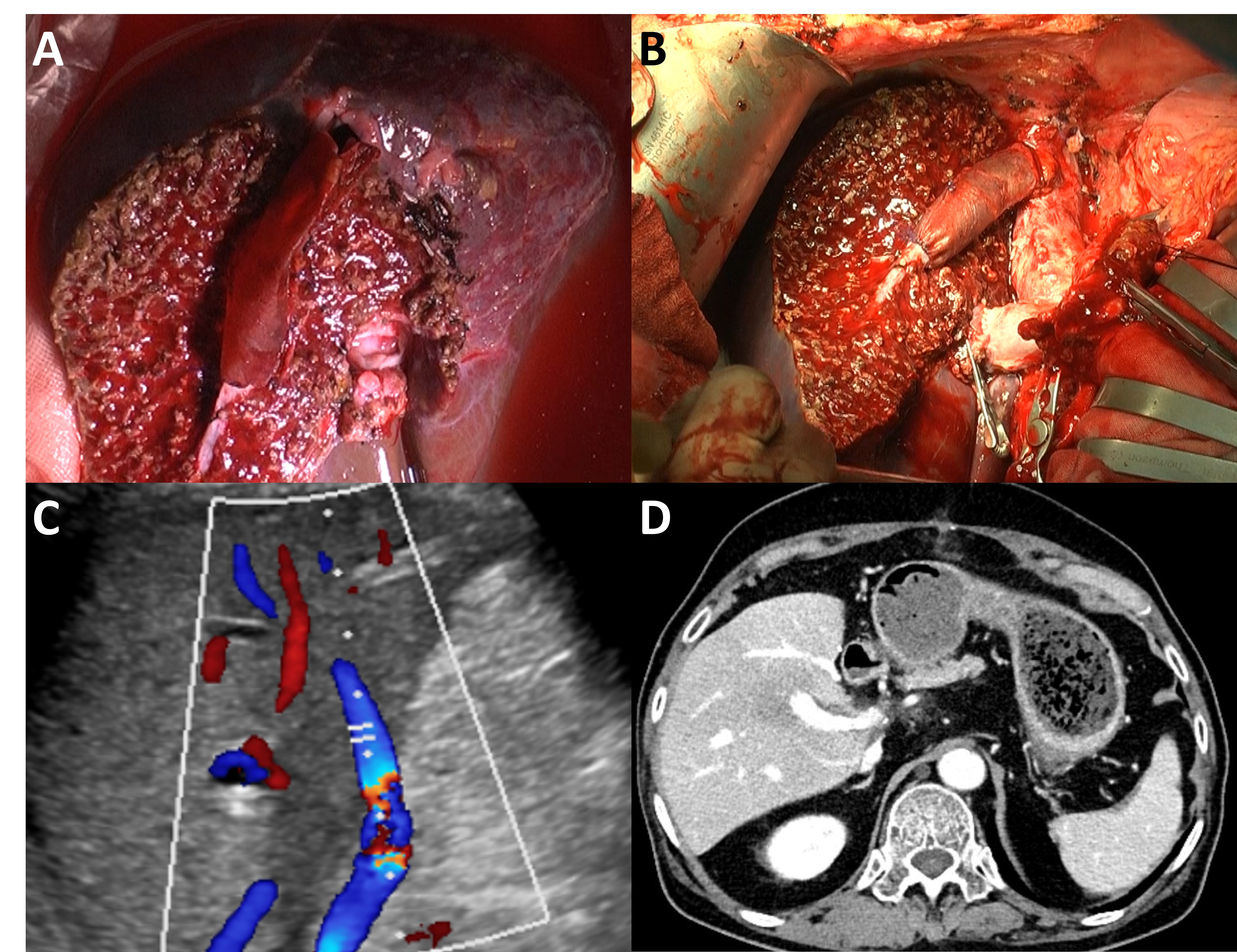
Results: The 1-,2-, 3-, and 5-month patency rates were 57.1%, 57.1%, 57.1%, and 28.6%, respectively. Among the total 15 cases assessed, the most recent 6 cases showed patent graft flow until discharge with 1-,2-,3-, and 5-month patency rates of 80.0%, 80.0%, 80.0%, and 20.0%, respectively. All patients survived with tolerable liver function tests. There were no significant congestion-related problems, except for one patient who experienced MHV thrombosis requiring aspiration thrombectomy and stent insertion. There were no infection-related complications. All patients survived to the final follow-up, with a minimum follow-up duration of 8 months.
Conclusions: Parietal peritoneum may be a novel autologous substitute for MHV reconstruction during LDLT.
[1] Yi NJ, Suh KS, Cho YB, et al. The right small-for-size graft results in better outcomes than the left small-for-size graft in adult-to-adult living donor liver transplantation. World J Surg. 2008;32:1722–1730.
[2] Lee S, Park K, Hwang S, et al. Anterior segment congestion of a right liver lobe graft in living-donor. J Hepatobiliary Pancreat Surg. 2003;10:16–25.
[3] Gyu Lee S, Min Park K, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation. 2002;74:54–59.
[4] Yi NJ, Suh KS, Lee HW, et al. An artificial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl. 2007;13:1159–1167.
[5] Sugawara Y, Makuuchi M, Akamatsu N, et al. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transpl. 2004;10:541–547.
[6] Hwang S, Lee SG, Ahn CS, et al. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 2005;11:644–649.
[7] Dong G, Sankary HN, Malagò M, et al. Cadaver iliac vein outflow reconstruction in living donor right lobe liver transplantation. J Am Coll Surg. 2004;199:504–507.
[8] Lee KW, Lee DS, Lee HH, et al. Interpostion vein graft in living donor liver transplantation. Transplant Proc. 2004;36:2261–2262.
[9] Cattral MS, Greig PD, Muradali D, et al. Reconstruction of middle hepatic vein of a living-donor right lobe liver graft with recipient left portal vein. Transplantation. 2001;71:1864–1866.
[10] Cekirdekci A, Bayar MK, Yilmaz S, et al. Reconstruction of the vena cava with the peritoneum: the effect of temporary distal arteriovenous fistula on patency (an experimental study). Eur J Vasc Endovasc Surg. 2004;27:84–88.
[11] Salimi F, Hodjati H, Monabbati A, et al. Inferior vena cava reconstruction with a flap of parietal peritoneum: an animal study. Arch Iran Med. 2009;12:448–453.
[12] Ribbe EB, Alm P, Hallberg E, et al. Evaluation of peritoneal tube grafts in the inferior vena cava of the pig. Br J Surg. 1988;75:357–360.
[13] Chin PT, Gallagher PJ, Stephen MS. Inferior vena caval resection with autogenous peritoneo-fascial patch graft caval repair: a new technique. Aust N Z J Surg. 1999;69:391–392.
[14] Akimaru K, Onda M, Tajiri T, et al. Reconstruction of the vena cava with the peritoneum. Am J Surg. 2000;179:289–293.
[15] Dokmak S. Pancreaticoduodenectomy with reconstruction of the mesentericoportal vein by the parietal peritoneum: ‘Safi Dokmak Vascular Graft.’ Ann Surg Oncol. 2015;22:343–344.
[16] Dokmak S, Aussilhou B, Calmels M, et al. Laparoscopic pancreaticoduodenectomy with reconstruction of the mesentericoportal vein with the parietal peritoneum and the falciform ligament. Surg Endosc. 2018;32:3256–3261.
[17] Pulitano C, Crawford M, Ho P, et al. Autogenous peritoneo-fascial graft: a versatile and inexpensive technique for repair of inferior vena cava. J Surg Oncol. 2013;107:871–872.
[18] Dokmak S, Aussilhou B, Sauvanet A, et al. Parietal peritoneum as an autologous substitute for venous reconstruction in hepatopancreatobiliary surgery. Ann Surg, 2015;262:366–371.
[19] Elias D, Honoré C, Dumont F, et al. Autologous peritoneo-fascial graft: a technique for vascular reconstruction. J Visc Surg. 2014;151:461–464.
[20] Yilmaz S, Kayaalp C, Battaloglu B, et al. Hepatic vein stenosis developed during living donor hepatectomy and corrected with peritoneal patch technique: a case report. Transplant Proc. 2012;44:1754–1756.
[21] Kóbori L, Doros A, Németh T, et al. The use of autologous rectus facia sheath for replacement of inferior caval vein defect in orthotopic liver transplantation. Transpl Int. 2005;18:1376–1377.
[22] Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17:471–484.
[23] Clavien P a, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196.
[24] Ou QJ, Hermann RE. Hepatic vein ligation and preservation of liver segments in major resections. Arch Surg. 1987;122:1198–1200.
[25] Madden RL, Lipkowitz GS, Browne BJ, et al. A comparison of cryopreserved vein allografts and prosthetic grafts for hemodialysis access. Ann Vasc Surg. 2005;19:686–691.
[26] Shell DH 4th, Croce MA, Cagiannos C, et al. Comparison of small-intestinal submucosa and expanded polytetrafluoroethylene as a vascular conduit in the presence of gram-positive contamination. Ann Surg. 2005;241:995–1001.
[27] Jernigan TW, Croce MA, Cagiannos C, et al. Small intestinal submucosa for vascular reconstruction in the presence of gastrointestinal contamination. Ann Surg. 2004;239:733–740.
[28] Kim MJ, Kim HB, Han JK, et al. Injuries of adjacent organs by the expanded polytetrafluoroethylene grafts in the venoplasty of middle hepatic veins in living-donor liver transplantation: computed tomographic findings and possible risk factors. J Comput Assist Tomogr. 2011;35:544–548.
[29] Ribbe EB, Norgren LEH, Thörne JL, et al. Platelet aggregation on peritoneal tube grafts and double velour grafts in the inferior vena cava of the pig. Br J Surg. 1988;75:81–85.
[30] Theuer CJ, Bergamini TM, Theuer HH, et al.. Vena cava replacement with a peritoneum-lined vascular graft. ASAIO J. 1996;42:266–270.
[31] Liptak JM, Chin PT, Stephen MS, et al. Segmental reconstruction of the caudal vena cava using an autogenous tubular graft of the internal rectus abdominus sheath: a pilot study. Vet Res. 2007;1:1–11.