
Dr. Twombley is a Professor with Tenure in the Department of Pediatrics, DIvision of Pediatric Nephrology at the Medical University of South Carolina. She is the Chief of Pediatric Nephrology and the Medical Director of Pediatric Kidney Transplantation. She is on Council for the International Pediatric Transplantation Association (IPTA) and is Chair of the Education Committee for IPTA. Her research intersts include antibiody mediated rejection in children and she is one of two PIs on a multicenter study called PARAMOUr “PediAtric Renal AMr Outcomes” Study.
Significance of antibody clearance, treatment and pathology findings in antibody mediated rejection in pediatric kidney transplant recipients on 1 year outcomes: Preliminary findings from the PARAMOUr “PediAtric Renal AMr Outcomes” study
Isa Ashoor3, Sonia Solomon4, Rachel Engen6, Amrish Jain2, Rouba Garro5, Mahmoud Kallash7, Katherine Twombley1.
1Pediatrics, Medial University of South Carolina, Charleston, SC, United States; 2Pediatrics, Wayne State University School of Medicine, Detroit, MI, United States; 3Pediatrics, Louisiana State University , New Orleans, LA, United States; 4Pediatrics, New York Medical College, Valhalla, NY, United States; 5Pediatrics, Emory University School of Medicine, Atlanta, GA, United States; 6Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, United States; 7Pediatrics, The Ohio State University, Columbus, OH, United States
PARAMOUr Study.
Background: Little is known about how donor specific antibody (DSA) clearance or pathology findings correlate with outcomes in pediatric antibody mediated rejection (AMR). There is also little know about how different treatments affect outcomes in pediatric AMR as there is no definied treatment to date.
Methods: We have collected data from 7 centers within the Pediatric Nephrology Research Consortium. Pediatric renal transplant recipients between the ages of 0 to 18 years at time of transplant old who had biopsy proven AMR between 12/31/2009-12/31/2017 and followed for 12 consecutive months were included. DSA clearance was defined as the percentage that the DSA decreased within a year post AMR treatment. P values for race, gender and donor type were obtained by comparing to SRTR data.
Microvascular Injury Score (MVI) = g+ptc
Chronic inflammation (CI) = ci+ct+cv+(cgX2)
Acute Inflammation (AI)= g+ptc+C4d
Interstitial Fibrosis and Tubular Atrophy (IFTA)= ci+ct
Results: We have DSA data on 37 AMR episodes and pathology data on 42 AMR episodes to date.
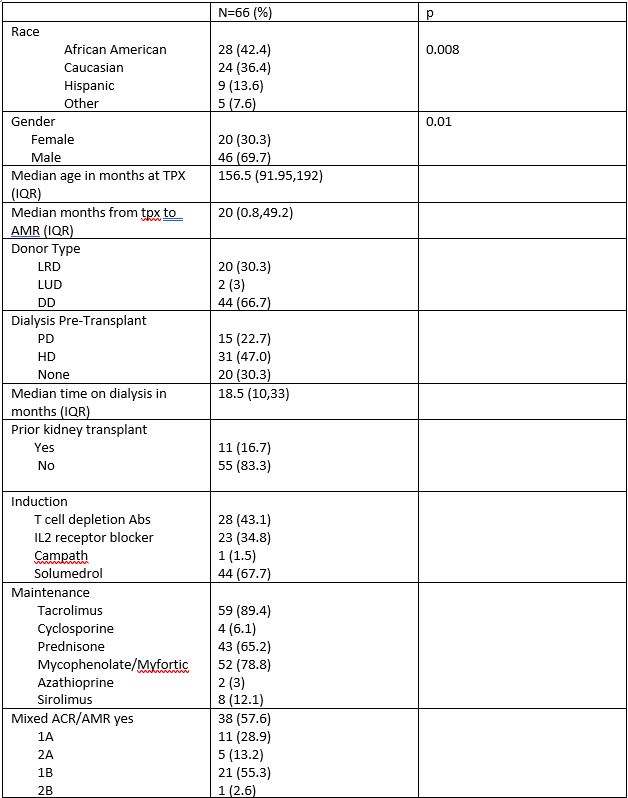
Specific treatment data is available for 66 AMR episodes to date.
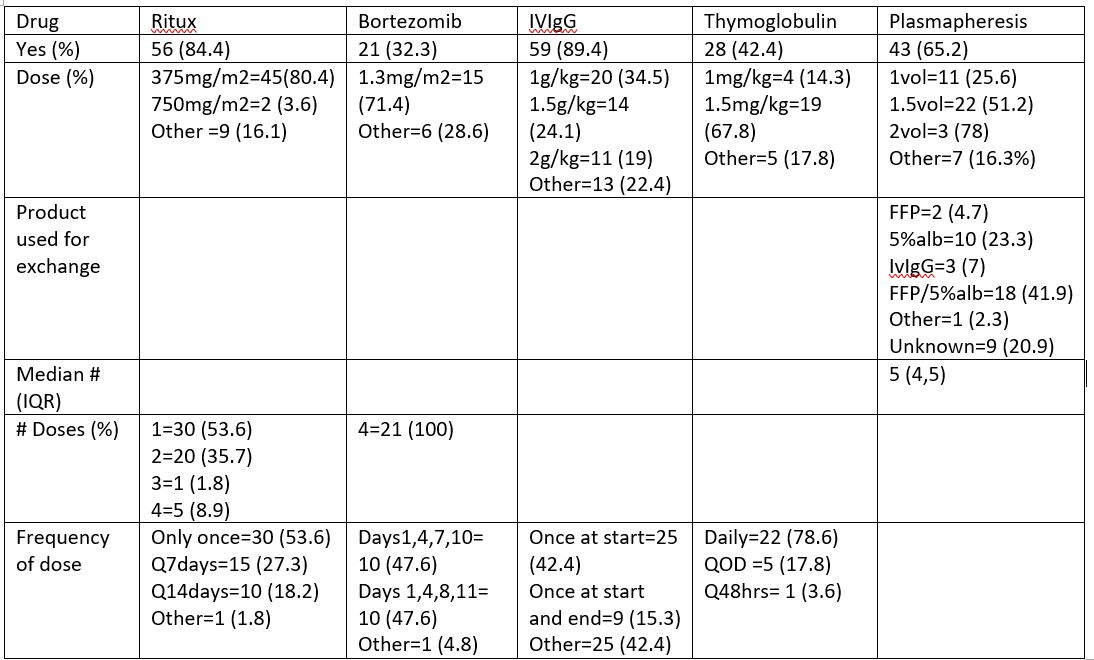
Class II was the immunodominant DSA (iDSA) in 81.1% (30/37) and Class I was iDSA in 19.9% (9/37). DQ was the most common iDSA 64.9% (24/37). On average only 27.38% of iDSA was cleared post treatment. There was no difference in the % of iDSA cleared between those that received bortezomib vs. no bortezomib (36.95% vs. 40.21%), or between those that received thymoglobulin vs. no thymoglobulin (30.68% vs. 41.68%). There was no difference in 1 yr GFR between those that cleared >50% of the iDSA vs <50%.
When looking at pathology, 52.4% (22/42) were mixed AMR/ACR. A good percentage of patinets, 26.2% (11/42) lost their graft by 1 year post AMR treatment. Of those who lost the graft, 27.3% (3/11) were pure AMR, and the others were mixed cellular and AMR.
36 samples were available for further Banff analysis to date.
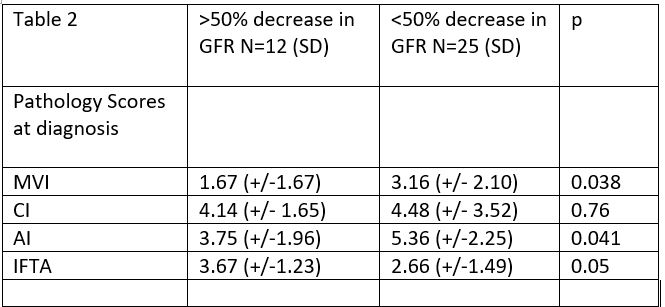
IFTA comparison: no difference in GFR at 1 year between those with IFTA >25% or less than 25%. (40.1 vs 29.5, p=0.17), MVI >2 vs. <2 (34.7 vs. 34.9; p=0.98), CI score >4 or <4 (41.4 vs 30.1; p=0.15), or AI >6or <6 (33.2 vs. 35.9; 9=0.73).
Discussion: Class II DSA are the most common iDSA seen in Pediatric AMR and DQ was the most common class II iDSA seen in our cohort. There was no correlation between clearance of iDSA and 1 year GFR. There was no difference in the iDSA clearance between medications used to treat AMR. Those with more chronic changes (IFTA) and less acute changes (MVI and AI) did had more GFR loss 1 year post treatment. We are still enrolling patients and collecting data from current patients. Larger numbers will hopefully confirm our current findings.
Pediatric Nephrology Research Consortium.
[1] Kizilbash S. Bortezomib in the treatment of antibody-mediated rejection in pediatric kidney transplant recipients: A multicenter Midwest Pediatric Nephrology Consortium study. Pediatric Transplantation 2017 May;21(3).
[2] Twombley K. Acute antibody-mediated rejection in pediatric kidney transplants: a single center experience. Pediatric Transplantation2013 Nov;17(7):E149-55.
There are no comments yet...