Long-term outcomes in kidney transplant recipients converting from twice-daily, immediate-release tacrolimus to once-daily, prolonged-release tacrolimus in Asian countries: 3-year interim analysis of the multicentre, non-interventional CHORUS study
Jaeseok Yang1, Yoshihiko Watarai2, Swapneel Anaokar3, Martin Blogg3.
1Department of Surgery, Seoul National University Hospital, Seoul, Korea; 2Department of Transplant Surgery, Nagoya Daini Red Cross Hospital, Nagoya, Japan; 3Astellas Pharma Europe, Ltd., Addlestone, United Kingdom
The CHORUS Study .
Introduction: Converting kidney transplant recipients (KTRs) from twice-daily, immediate-release tacrolimus (IRT) formulations to once-daily, prolonged-release tacrolimus (PRT) may improve medication adherence, decrease variability in tacrolimus exposure and provide more stable renal function. Long-term data on transplant outcomes following conversion are lacking in Asian KTRs. We present an interim subgroup analysis of the CHORUS study at 3 years after conversion from IRT to PRT in KTRs recruited in Asian countries.
Materials and Methods: CHORUS is a 5-year, multicentre, non-interventional study (NCT02555787) in adult KTRs converting from IRT to PRT (Advagraf®, Astellas Pharma Europe, Ltd) under routine practice conditions. The primary endpoint was change in renal function (eGFR, calculated by Modified Diet in Renal Disease 4). Tacrolimus dose and trough levels, Kaplan–Meier estimated rates of biopsy-confirmed acute rejection (BCAR)-free, graft and patient survival, and safety were also assessed. Analyses were stratified by time of conversion post-transplant (early, ≤6 months; late, >6 months).
Results: Of the 4372 KTRs enrolled in CHORUS, 907 (20.7%) were recruited in Asian countries; 884 formed the full analysis set (early converters, 91; late converters, 793). Baseline demographics were similar between groups, except for a higher proportion of males in the early conversion group (70.3% vs 57.5% in the late conversion group); overall mean (SD) age was 49.6 (11.4) years. Median (range) time from transplantation to conversion was 3.4 (0.2–5.9) months for early converters vs 42.1 (6.1–369.0) months for late converters. Mean PRT dose was 4.00 (2.20) vs 3.11 (1.61) mg/day at conversion and 3.05 (1.29) vs 3.47 (1.91) mg/day at 36 months after conversion, for early vs late converters, respectively (Table 1).
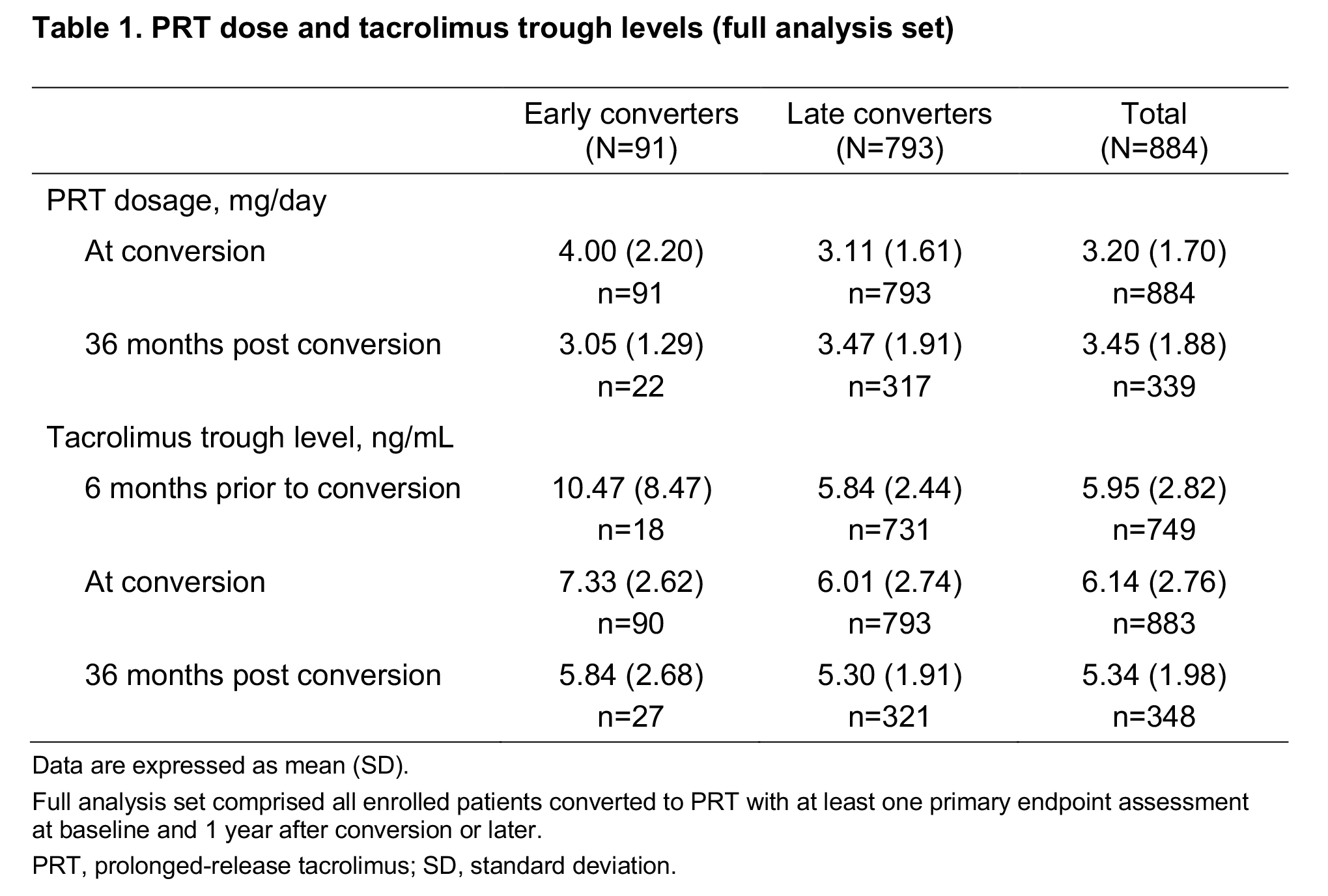
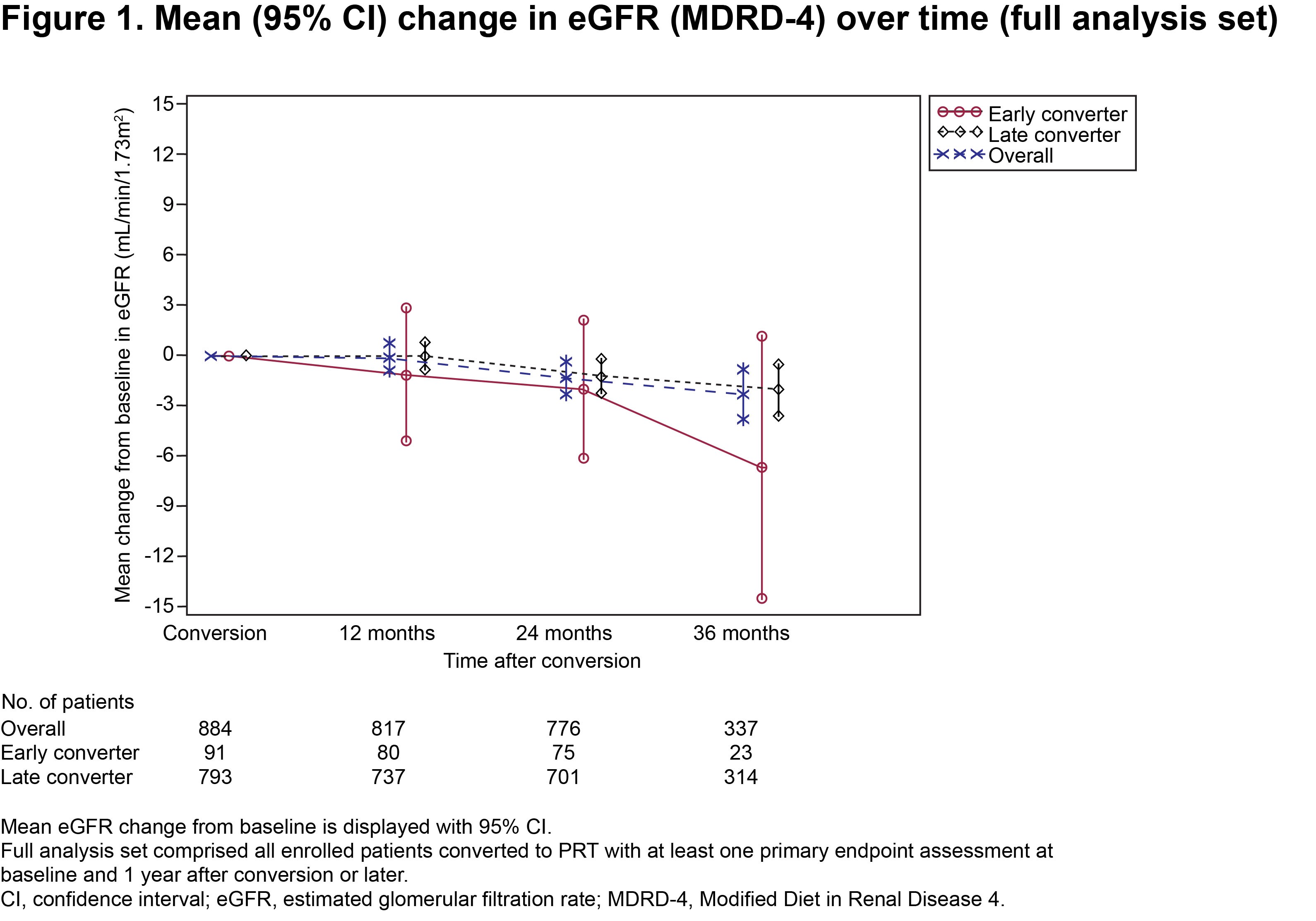
Respective mean duration of PRT treatment was 755.8 (287.8) vs 847.5 (264.3) days. Mean tacrolimus trough levels were 7.33 (2.62) vs 6.01 (2.74) ng/mL at conversion and 5.84 (2.68) vs 5.30 (1.91) ng/mL at 36 months, in the two groups, respectively (Table 1). Mean (SE) change in eGFR from time of conversion to 36 months post conversion was −6.69 (3.77) mL/min/1.73m2 in early converters vs −2.01 (0.80) mL/min/1.73m2 in late converters (Fig. 1).
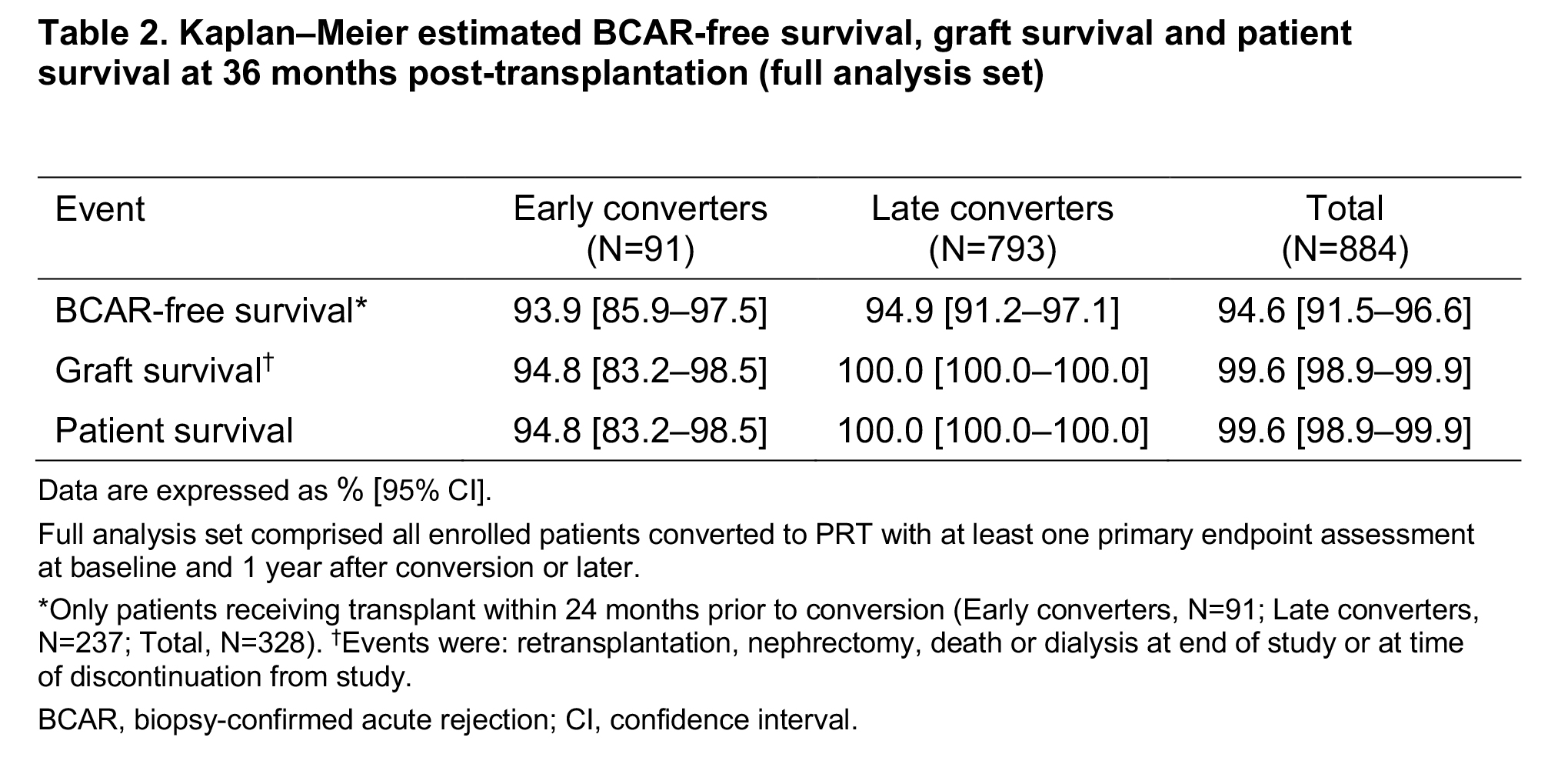
Kaplan–Meier estimated BCAR-free survival, graft survival and patient survival rates were similar between groups at 36 months post transplant (Table 2).
Safety was assessed in all patients (early converters, 98; late converters, 809). Adverse events (AEs) were reported by 44.9% of early converters vs 41.8% of late converters, and were considered treatment-related in 9.2% vs 6.7%. Respective incidences of treatment-related serious AEs were 7.1% vs 3.8%.
Conclusion: In this sub-cohort of KTRs in Asian countries, consistent outcomes were observed at 3 years after conversion from IRT to PRT. Relevant clinical differences will be assessed at end of study after 5 years of follow up.
Astellas Pharma Europe Ltd.
There are no comments yet...