Diagnostic tests for delayed graft function: A systematic review
Christina Lai1,2, Seow Yeing Yee3, Tracey Ying1,2, Steve Chadban1,2.
1Renal Medicine, Royal Prince Alfred Hospital, Camperdown, Australia; 2Kidney Node Laboratroy, The University of Sydney, Camperdown, Australia; 3Nephrology Department, Hospital Kuala Lumpur, Wilayah Persekutuan Kuala Lumpur, Malaysia
Background: Delayed graft function (DGF) is associated with inferior graft and patient survival and is frequently used in clinical trials as a surrogate outcome. DGF is most commonly defined as the requirement for dialysis within 7-days post-transplant, however the indication for dialysis is often subjective. Diagnosis of DGF using biomarkers or other tests may provide greater objectivity, enable earlier diagnosis and permit gradation of severity. The aim of this study was to perform a systematic review of novel tests and biomarkers that have been used to diagnose DGF.
Methods: We performed a systematic literature search from inception to December 2018 using MEDLINE and EMBASE. We included all prospective and retrospective cohort studies in kidney transplant recipients, where a diagnostic test was used to diagnose DGF and compared to the gold standard definition of requirement for dialysis within 7 days post-transplant. The reporting quality for each article was evaluated according to the Standards for Reporting of Diagnostic Accuracy (STARD) and risk of bias was assessed using Quality Assessment of Diagnostics Accuracy Studies 2 tool as outlined by the Cochrane Collaboration. We prospectively developed a protocol for this systematic review and registered it with PROSPERO, an international prospective register of systematic reviews (Registration number: CRD42018108856).
Results: From 3,309 citations we identified 37 studies including 1916 kidney transplant recipients. Twenty novel diagnostic biomarkers were identified (Table 1). The number of participants in each study ranged from 16 to 138 patients (mean=52 patients). The most common tests were serum NGAL (8 studies; 357 participants) and urine NGAL (7 studies; 387 participants). The threshold used to determine positive results varied among studies. Only one study reported an independent association of biomarker with 1-year graft outcome. The majority of studies were single-centre studies (33/37, 89%). The overall reporting quality of the studies was low, and the risk of bias was unclear. Seventy-three percent of studies did not report blinding for index or reference tests.
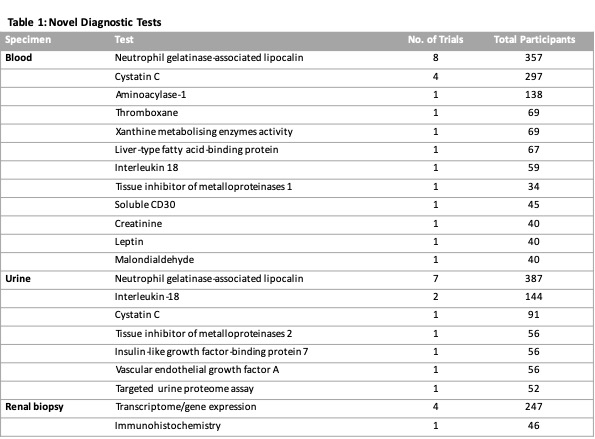
Discussion: The results of our systematic review have not identified a superior test. Most studies included in the review were exploratory and further validation studies are required to confirm the role of individual diagnostic test. Our findings highlight the need for a larger study comparing the strength of association between the current gold standard and alternative diagnostic criteria with short- and long-term outcomes.
Conclusion: Novel methods for diagnosing DGF remain exploratory and a superior test to the conventional definition of requirement for dialysis within seven days of transplantation could not be identified. Larger studies capturing long term outcomes will be required to determine the predictive value of any novel diagnostic test for DGF.
Christina Lai is funded by Postgraduate Research Scholarship in Renal Medicine from the University of Sydney for her postdoctoral studies. .