Targeting inflammatory monocytes by immune-modifying nanoparticles prevents acute kidney allograft rejection
Christina Lai1,2,3, Steve Chadban1,2,3, Julian Singer1,2,3, Yik Wen Loh1,2,3, Paula Niewold3,4, Daniel Getts3,4, Nicholas King3,4, Huiling Wu1,2,3.
1Renal Medicine, Royal Prince Alfred Hospital, Camperdown, Australia; 2Kidney Node Laboratory, The University of Sydney, Camperdown, Australia; 3The Charles Perkins Centre, The University of Sydney, Camperdown, Australia; 4The Discipline of Pathology, School of Medical Sciences, The University of Sydney, Camperdown, Australia
Aim: We previously reported the capacity of immune-modifying nanoparticles (IMPs) to bind to circulating inflammatory monocytes/macrophages (MΦ) via the specific scavenger receptor MARCO, thereby causing MΦ removal in the spleen with subsequent protection in models of infection, autoimmunity and ischemia-reperfusion injury (IRI). Here we investigated the therapeutic potential of IMPs to target MΦ in kidney allograft rejection in a murine model of kidney transplantation.
Methods: Kidney transplants were performed from BALB/c to C57BL/6 mice as WT allografts. A group of WT allograft mice received a daily intravenous injection of 300µl (1.46x1010particles/ml, 500nm in diameter) of negatively-charged fluorescein isothiocyanate (FITC)–labelled IMPs (FITC-IMPs) from day 1 post-transplant for two weeks (WT+IMP). Samples were collected at days 14 and 100 post-transplant to access acute and chronic allograft rejection.
Results and Discussion: WT+IMP allografts had prolonged survival compared to WT allografts (Figure 1, p<0.05). At day 14 post-transplant, WT+IMP allografts were protected from acute rejection with lower creatinine (23.8±1.4 vs. 48.1±18.7mmol/L, p<0.01) and less tubulitis (95.6±12.6 vs. 140.4±33.9scores, p<0.001)(Figure 2). Histologically, accumulation of CD4+ (38±13 vs. 73±32 cells/HPFs, p<0.01), CD8+ (39±18 vs. 76±39 cells/HPFs, p<0.05), CD68+ (12±5 vs. 22±7% field positive, p<0.01) and CD11c (2±1 vs. 13±5% field positive, p<0.001) cells were reduced in WT+IMP allografts, compared to WT allografts. High dimensional flow cytometry analysis of cells isolated from allografts showed significantly reduced T cells and myeloid cells in WT+IMP. By immunohistology double staining with F4/80, MARCO and caspase-3, FITC-IMPs were found to predominantly co-localize with F4/80-expressing red pulp macrophages that expressed MARCO and caspase-3, suggesting that IMPs divert MΦ to the spleen, undergoing caspase-3–mediated apoptosis (Figure 3). WT+IMP allografts expressed significantly less pro-inflammatory (IL6) and Th1 (IFNγ) cytokines and cytotoxic molecules (perforin, granzyme B & iNOS). At day 100 post-transplant, WT+IMP allografts exhibited chronic allograft injury with comparable levels of serum creatinine (49.8±34.6 vs. 48.8±26.8mmol/L, p>0.99) and interstitial fibrosis by collagen deposition compared to WT allografts.
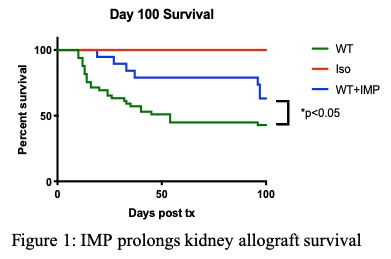
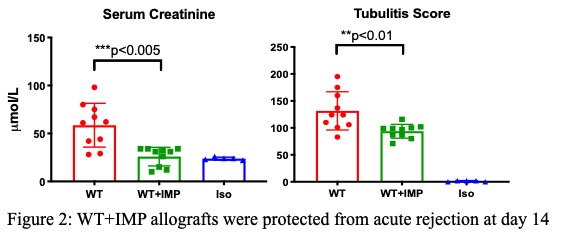
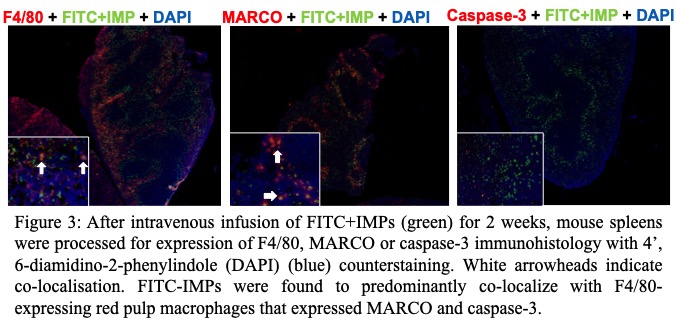
Conclusion: IMP affords significant protection from acute allograft rejection. IMP infusion caused inflammatory MΦ sequestration in the spleen through apoptotic cell clearance mechanisms and, ultimately, caspase-3–mediated apoptosis. Coupled with our earlier demonstration of IMP-induced protection in kidney IRI, the additional ability to retard acute rejection identifies IMP as a potential induction agent in kidney transplantation. As both delayed graft function and acute rejection remain unmet needs in the clinic, translational studies using IMP warrant further consideration.
Christina Lai is funded by Postgraduate Research Scholarship in Renal Medicine from the University of Sydney for her postdoctoral studies.