
Clazakizumab (anti-IL-6) induces FoxP3+ Tregs in highly HLA sensitized patients receiving HLAi kidney transplantation (NCT03380962)
Stanley Jordan1, Shili Ge2, Nori Ammerman1, Mieko Toyoda2, Edmund Huang1, Alice Peng1, Reiad Najjar1, Supreet Sethi1, Summer Williamson1, Kate Myers1, Kathlyn Lim1, Jua Choi1, Ashley A. Vo1.
1Kidney Transplant, Cedars Sinai Medical Center, Los Angeles, CA, United States; 2Transplant Immunology Laboratory, Cedars Sinai Medical Center, Los Angeles, CA, United States
Introduction: Interleukin-6 is an important inflammatory cytokine which also acts as a growth factor for B-cells, plasma cells and Th17 cells. IL-6 also inhibits FoxP3+ Treg cells. These considerations suggest blocking IL-6 may be an important method to reduce inflammation and Th17 mediated injury in kidney allografts.
Patients & Methods: Clazakizumab (Vitaeris Inc.) is a humanized monoclonal antibody aimed at the cytokine IL-6. As part of a phase I/II trial of clazakizumab for desensitization, HLA sensitized patients received anti-IL-6, 25mg SC monthly X 6 doses with monitoring of HLA antibody levels and Tregs. Patients were treated pre- and post-transplant with anti-IL-6. Transplanted patients received monthly claza 25mg SC starting 5-7 days post-transplant for 12M. Tregs were determined by flow cytometry as CD4+,CD25+,CD127dim,FoxP3+ cell populations in CD4+ cells. Determinations were made at baseline, at transplantation and day 180 post-transplant.
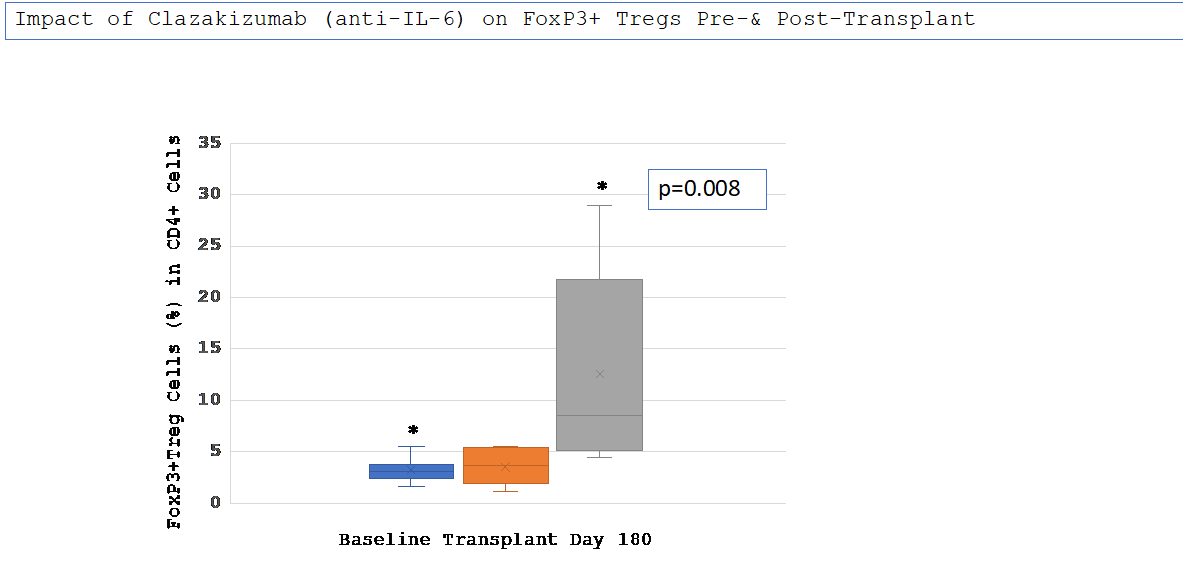
Results: Nine patients were transplanted. All patients had previous transplants; 78% had cPRA 99-100%, 67% were B-cell FCMX+ and class II DSA+ @ time of transplant. Mean MFI for HLA cI & cII were: pre-desensitization vs. post claza: cI 13062±3123 vs. 8585±4597 (p=0.05) and cII 13519±2966 vs. 8344±4836 (p =0.03). All DSA+ patients were negative by day 180 post-transplant. Mean Treg values at baseline v. at transplant did not differ (3.2+1.09% v.3.5+1.75%,p=NS). However, were significantly different at day 180 post-transplant (3.2+1.09% v.3.5+1.75% v. 12.6+9.3%, p=0.008)(Figure 1).
Conclusions: Clazakizumab desensitization reduced HLA cI/cII antibodies and allowed 9/10 highly sensitized patients to receive transplants. In addition, a dramatic increase in Treg cells at day 180 post-transplant was seen while patients were still on anti-IL-6 therapy suggesting that anti-IL-6 may induce CD4+ T-cells toward a Treg profile. This may have therapeutic implications for modifying baseline immunosuppression post-transplant.
Vitaeris, Inc..