Evaluation of the mechanism of action of hepatic hypothermic oxygenated machine perfusion (HOPE) using microdialysis in a clinical setting
Damiano Patrono1, Anna Teresa Mazzeo2,3, Dorotea Roggio4, Giorgia Catalano1, Federica Rigo1, Vito Fanelli2, Renato Romagnoli1.
1General Surgery 2U – Liver Transplant Unit, A.O.U. Città della Salute e della Scienza di Torino - University of Turin. , Turin, Italy; 2Department of Surgical Science, Anaesthesia and Critical Care, A.O.U. Città della Salute e della Scienza di Torino - University of Turin. , Turin, Italy; 3Department of Adult and Pediatric Pathology. Messina, Italy, Anesthesia and Intensive Therapy Unit – University of Messina. , Messina, Italy; 4Department of Molecular Biotechnology and Health Sciences, University of Turin, Turin, Italy
Introduction: HOPE is progressively entering the clinical practice, but the understanding of its mechanism of action is still incomplete. Microdialysis (MD) offers the possibility to sample interstitial fluid during both static cold storage (SCS) and HOPE.
Materials and Methods: Dual HOPE was carried out as described by Groningen group. A 40/30 63 MD catheter (membrane cutoff 20 kDa) was inserted in segment 6 liver parenchyma and perfused at 2 μl/min with normal saline through a precision MD pump during backtable preparation and subsequent HOPE in 11 patients. Glucose, lactate, pyruvate, glutamate, NADH and FMN levels were measured on samples collected every hour (T1: backtable; T2: HOPE 1sthour; T3: HOPE 2ndhour; T4: HOPE 3rdhour). Similar analyses were conducted on perfusate samples collected every 30 min during HOPE. Liver biopsies were obtained before and after backtable, after HOPE and after graft reperfusion into recipient.
Results and Discussion: Mean (SD) cold ischemia and HOPE time were 360 (47) 118 (29) minutes. Overall, there was an increase in MD mean (SD) glucose during HOPE as compared to SCS (5.34 [2.05] versus 13.4 [4.61] mmol/L, p=0.001; Figure 1), whereas lactate, pyruvate and glutamate levels were stable.
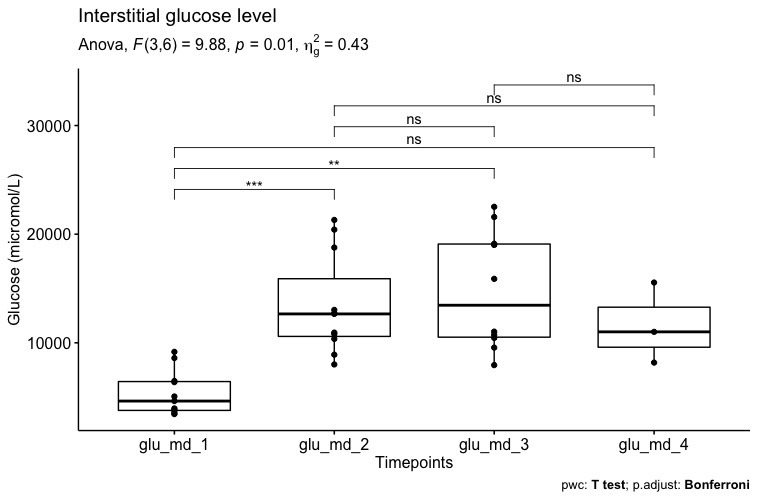
Lactate/pyruvate (L/P) ratio was constantly high as a result of low pyruvate level. Patients developing early allograft dysfunction had a different MD glucose and lactate trend (Figure 2)
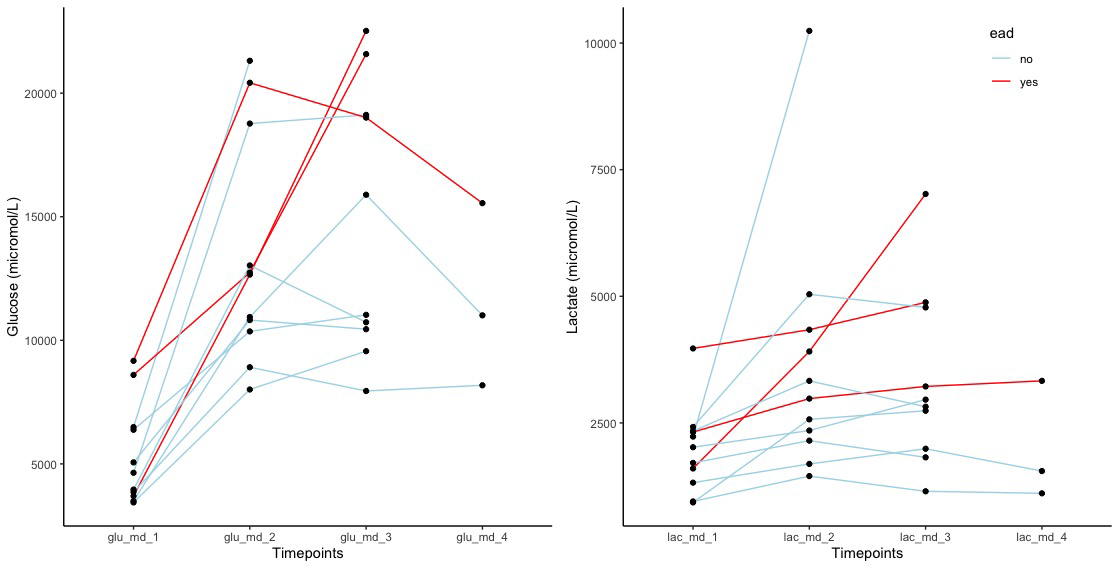
and higher glucose (21 [1.8] versus 12.1 [3.9]mmol/L,p=0.006) and lactate (5 [1.9] versus 2.6 [1.2] mmol/L,p=0.03) levels during HOPE 2ndhour (Figure 3).
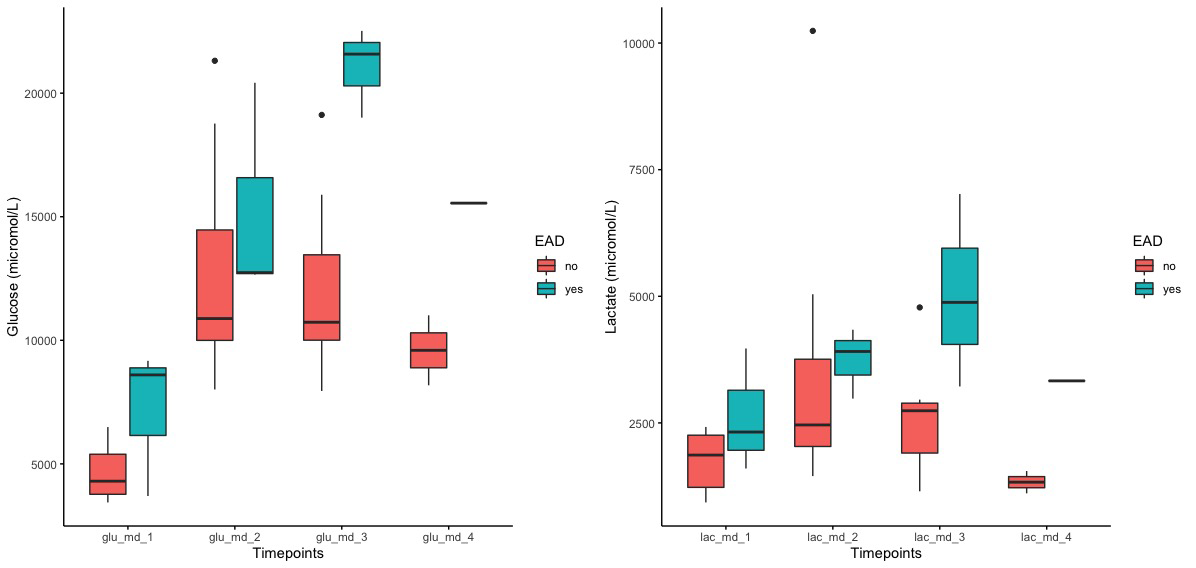
L/P had a similar but non-significant trend. Tissue levels of inflammatory cytokines (IL-6, IL-8, CXCL12, TNFα), adhesion molecules (ICAM-1) and HIF1α were stable during SCS and HOPE and increased upon graft reperfusion (Figure 4).
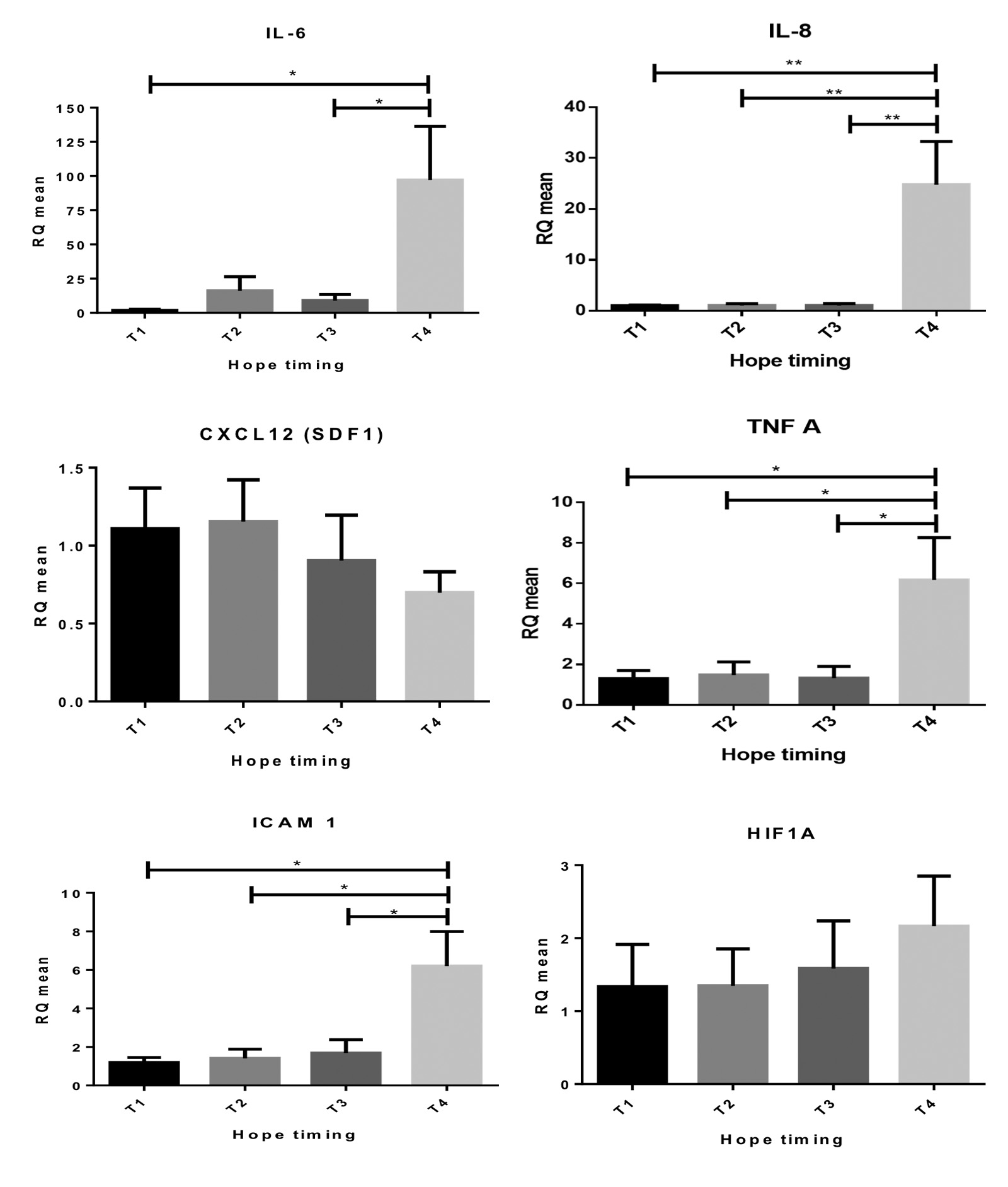
Expression of innate immune response markers (MYD88, TLR4) was stable at all timepoints.
Conclusion: This study provides new insight into hepatic metabolism during SCS and HOPE. Hepatic metabolism during HOPE and SCS remains stably low. HOPE is not associated with the expression of inflammatory markers or adhesion molecules. MD glucose and lactate level constantly increases during HOPE, with a pattern associated with subsequent EAD development, potentially representing an indicator of previous ischemia-reperfusion injury.