Cardiovascular outcomes of rapamycin-based immunosuppression in kidney transplant recipients: A single center retrospective study
Sojung Yoon1, Sangmi Lee2, Si Youn Kim1, Hyung Woo Kim2, Kyu Ha Huh3,4, Beom Seok Kim2,4.
1College of Medicine, Yonsei University, Seoul, Korea; 2Department of Internal Medicine, Severance Hospital, Seoul, Korea; 3Department of Surgery, Yonsei University College of Medicine, Seoul, Korea; 4The Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea
Introduction: In the past decade, the cause of death of recipients who underwent kidney transplantation (KT) has been changed, and cardiovascular disease (CVD) remains the leading cause of death followed by infection and malignancy in kidney transplant recipients. Rapamycin, commonly used mammalian target of rapamycin inhibitors (mTOR inhibitors) after KT for immunosuppression, increases the risk of atherosclerosis by causing hyperglycemia and hyperlipidemia, while also reducing the risk by its anti-proliferative and anti-inflammatory effect. This study aimed to address the cardiovascular risk depending on the load of the rapamycin treatment.
Methods: We retrospectively analyzed 239 patients who received more than a day of oral rapamycin after KT at Severance Hospital from January 2005 to December 2015. Depending on the duration of exposure to rapamycin, subjects were categorized into 3 groups with a 3-year increase. The primary endpoint was a major adverse cardiovascular event (MACE) defined as a composite of myocardial infarction, percutaneous coronary intervention or coronary artery bypass surgery, ischemic stroke, or all cause death.
Results:
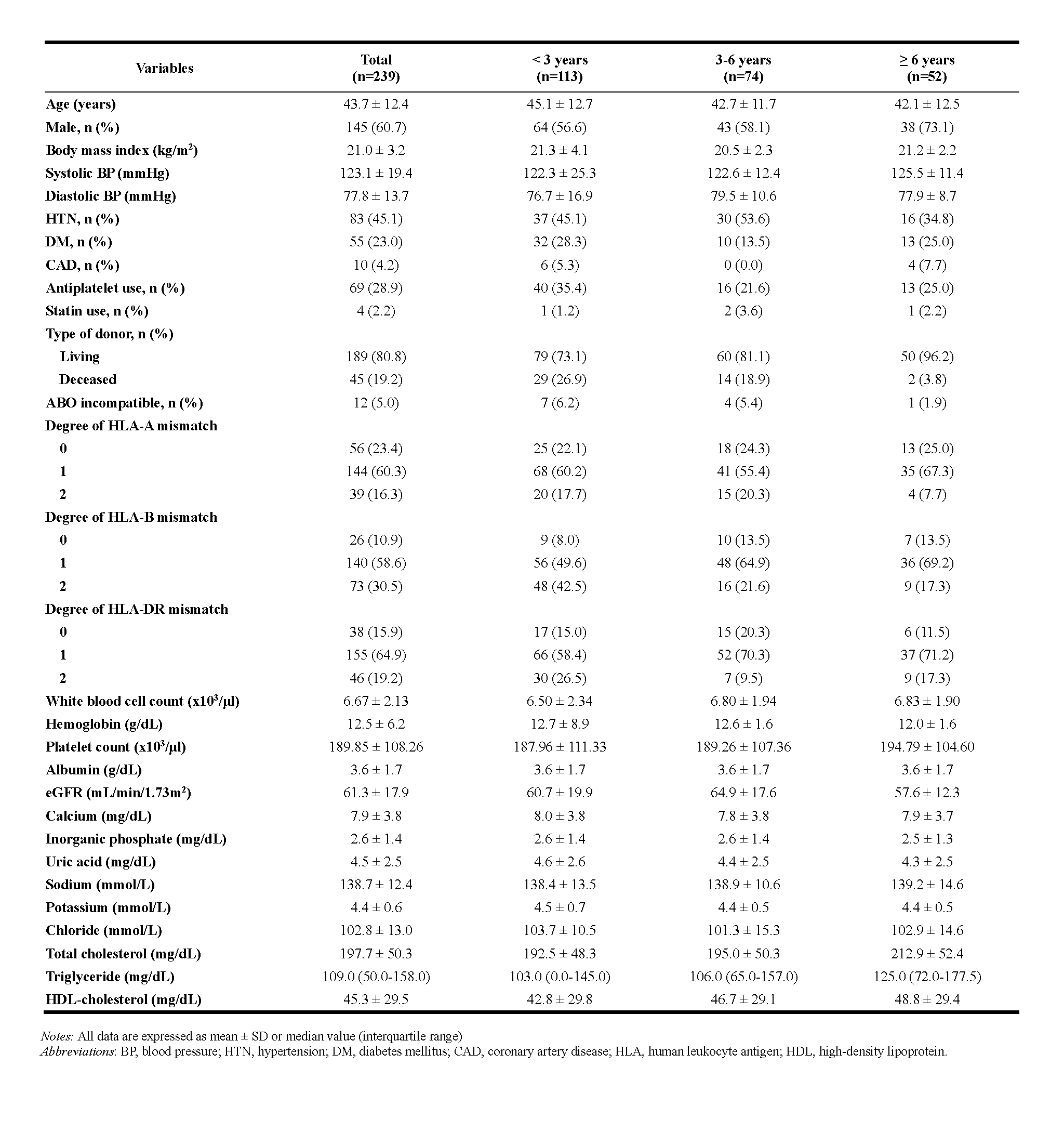
There were 113, 74 and 52 patients in the rapamycin exposure less than 3 years group, over 3 years and less than 6 years group, and over 6 years group, respectively. The KT recipients who were treated rapamycin longer had higher rate of living donor transplantation and lower HLA mismatch degree. The mean follow-up was 8.3 years and the incidence rates of MACE were 16.1, 6.6, and 9.7 events per 1000 persons-years in the shortest, middle, and the longest exposed group.
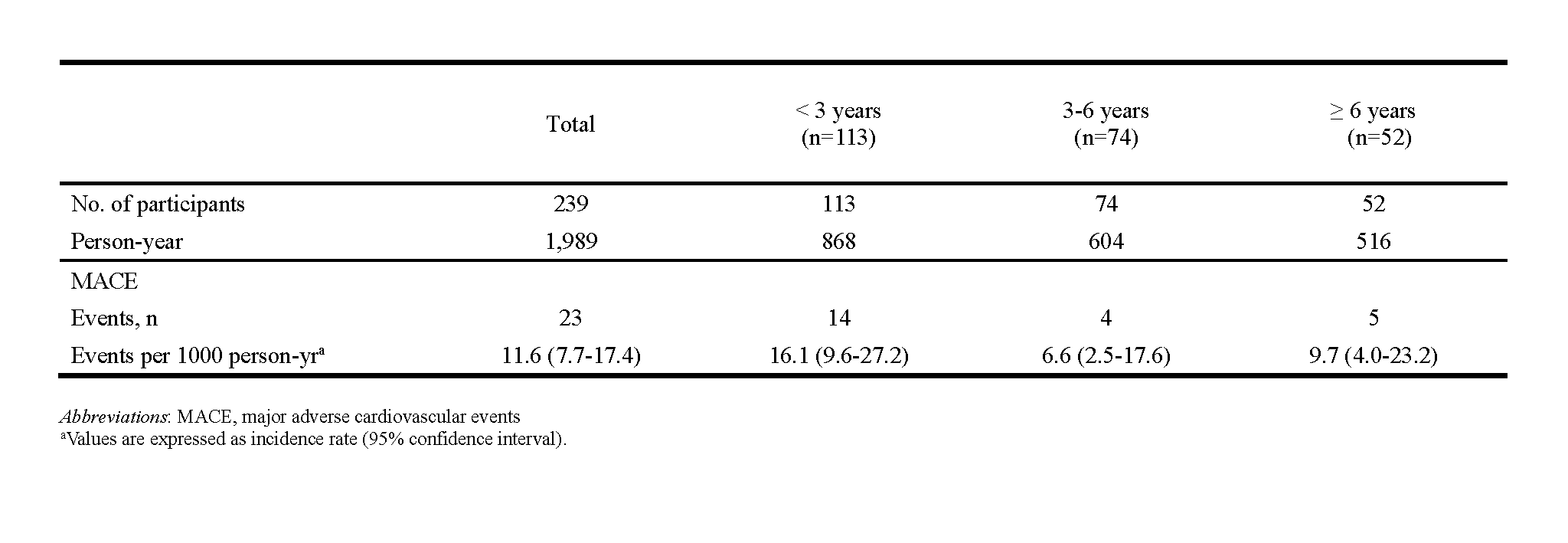
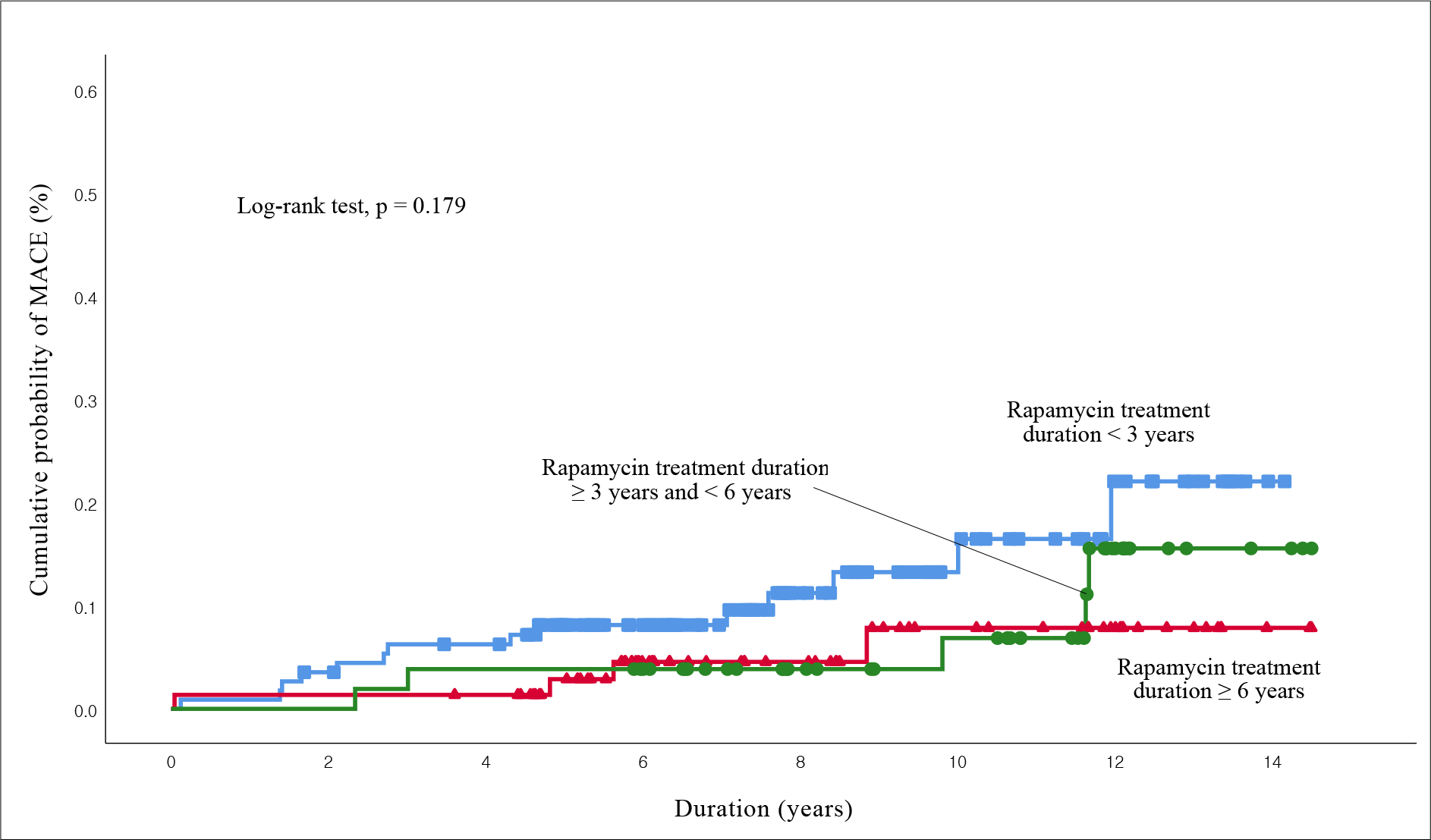
The risk of MACE decreased 0.74 fold for more than 3 years and less than 6 years group, and 0.68 fold for more than 6 years group adjusted with potential confounders, but it was not statistically significant (p = 0.673 and 0.577).
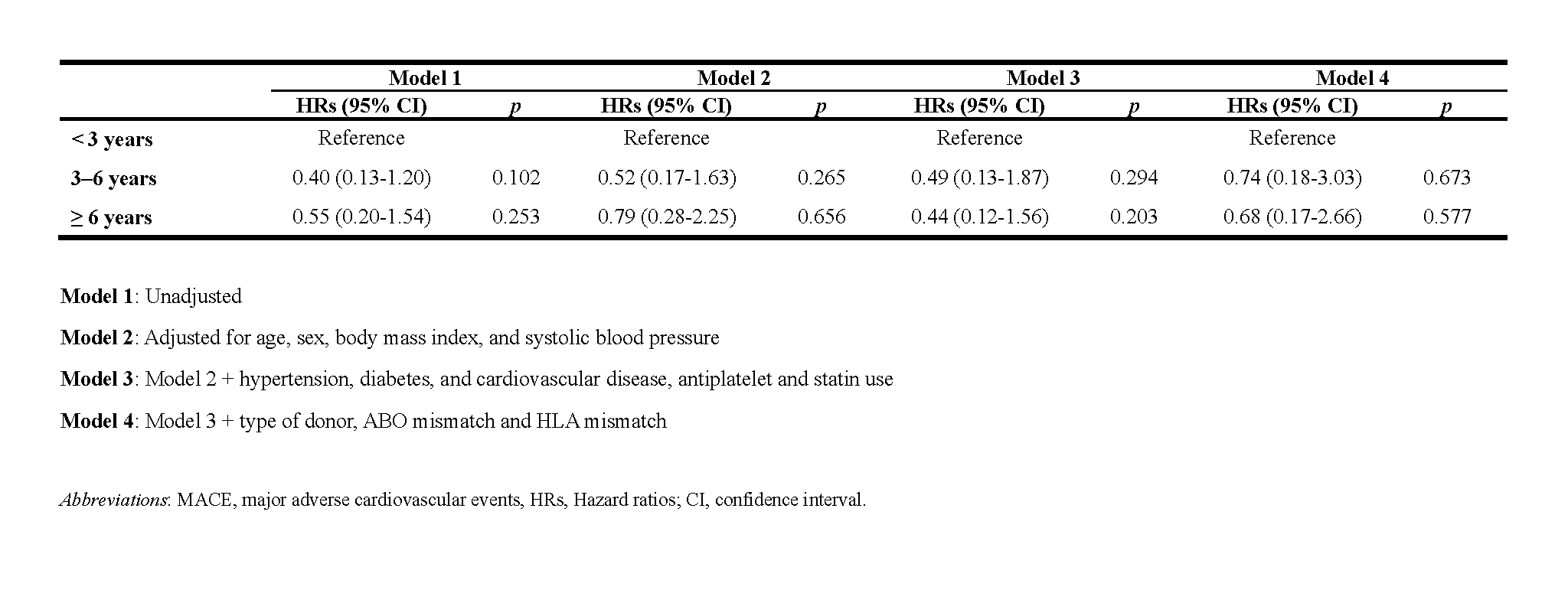
Discussion: This analysis of renal transplant recipients confirmed the MACE outcome according to the duration of rapamycin prescription at an interval of 3 years. Although no significant difference was obtained, the trend that cardiovascular risk decreases as the load of rapamycin increases could be confirmed. Notably, in this study, the mean follow-up duration was 8.3 years, which is a period enough to confirm the cardiovascular outcomes. Limitations of this study include that it was a retrospective analysis of a single arm cohort study with relatively small sample size. All potential confounding variables that are known to influence the cardiovascular outcome, the start and end date of rapamycin treatment and their correlation with the date of MACE, and the combination of other immunosuppressive agents were not controlled.
Conclusions: The risk of MACE tended to be lower as the duration of rapamycin exposure increased. Further studies with larger population will guide appropriate selection of immunosuppressants to prevent CVD.
This study was supported by the Student Research Fund of Yonsei University College of Medicine in 2018.. In this study, Sojung Yoon and Sangmi Lee contributed equally as the first author, and Beom Seok Kim and Kyu Ha Huh corresponded equally as the corresponding authors..
[1] Jeon HJ, Bae HJ, Ham YR, et al. Outcomes of end-stage renal disease patients on the waiting list for deceased donor kidney transplantation: A single-center study. Kidney Res Clin Pract. 2019;38(1): 116-123.
[2] Awan AA, Niu J, Pan JS, et al. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996-2014). Am J Nephrol. 2018;48(6): 472-481.
[3] Peddi VR, First MR. Recent advances in immunosuppressive therapy for renal transplantation. Semin Dial. 2001;14(3): 218-222.
[4] McKeage K, Murdoch D, Goa KL. The sirolimus-eluting stent: a review of its use in the treatment of coronary artery disease. Am J Cardiovasc Drugs. 2003;3(3): 211-230.
[5] Xie X, Jiang Y, Lai X, Xiang S, Shou Z, Chen J. mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrol. 2015;16: 91.
[6] Kasiske BL, de Mattos A, Flechner SM, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant. 2008;8(7): 1384-1392.
[7] Elloso MM, Azrolan N, Sehgal SN, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3(5): 562-569.
[8] Ciancio G, Burke GW, Gaynor JJ, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimums/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three-year analysis. Transplantation. 2006;81(6): 845-852.
[9] Chait A, Bornfeldt KE. Diabetes and atherosclerosis: is there a role for hyperglycemia? J Lipid Res. 2009;50 Suppl: S335-339.
[10] van Dijk M, van Roon AM, Said MY, et al. Long-term cardiovascular outcome of renal transplant recipients after early conversion to everolimus compared to calcineurin inhibition: results from the randomized controlled MECANO trial. Transpl Int. 2018;31(12): 1380-1390.
[11] Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2): 115-126.
[12] Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917): 868-874.
[13] Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5): 335-340.
[14] Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. Am J Transplant. 2002;2(6): 551-559.
[15] Ong HT. Beta blockers in hypertension and cardiovascular disease. BMJ. 2007;334(7600): 946-949.
[16] Baumhakel M, Bohm M. Cardiovascular outcomes with angiotensin II receptor blockers: clinical implications of recent trials. Vasc Health Risk Manag. 2011;7: 391-397.
There are no comments yet...