Near-single-cell proteomicsprofiling of the proximal tubular and glomerulus of the normal human kidney
Tara Sigdel1, Paul D. Piehowski2, Sudeshna Roy1, Juliane Liberto1, Joshua R. Hansen2, Adam C. Swensen2, Priyanka Rashmi1, Andrew Schroeder1, Izabella Damm1, Wei-Jun Qian2, Minnie M. Sarwal1.
1Surgery, University of California San Francisco, San Francisco, CA, United States; 2Biological Sciences Division and Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA, United States
Kidney Precision Medicine Project.
Introduction: Molecular assessments at the single cell level can accelerate biological research by providing detailed assessments of cellular organization and tissue heterogeneity in both disease and health. The human kidney has complex multi-cellular states with varying functionality, much of which can now be completely harnessed with recent technological advances in tissue proteomics at a near single-cell level. We discuss the foundational steps in the first application of this mass spectrometry (MS) based proteomics method for analysis of sub-sections of the normal human kidney, as part of the Kidney Precision Medicine Project (KPMP).
Materials and Methods: For this purpose, we used approximately 30-40 laser captured micro-dissected kidney cells to analyze proteins in glomerular and proximal tubular cells of kidney. A recently developed and optimized mass spectrometry based technology called "microPOTS" was used for cell specific protein expression. Enrichment analysis and "cross-omics" analysis was performed to validate the findings.
Results: We demonstrated that fresh frozen kidney tissues are optimal tissues for tsingle cell proteomics analysis. we identified more than 2500 human proteins, with specificity to the proximal tubular (PT; n=25 proteins) and glomerular (Glom; n=67 proteins) regions of the kidney and their unique metabolic functions.
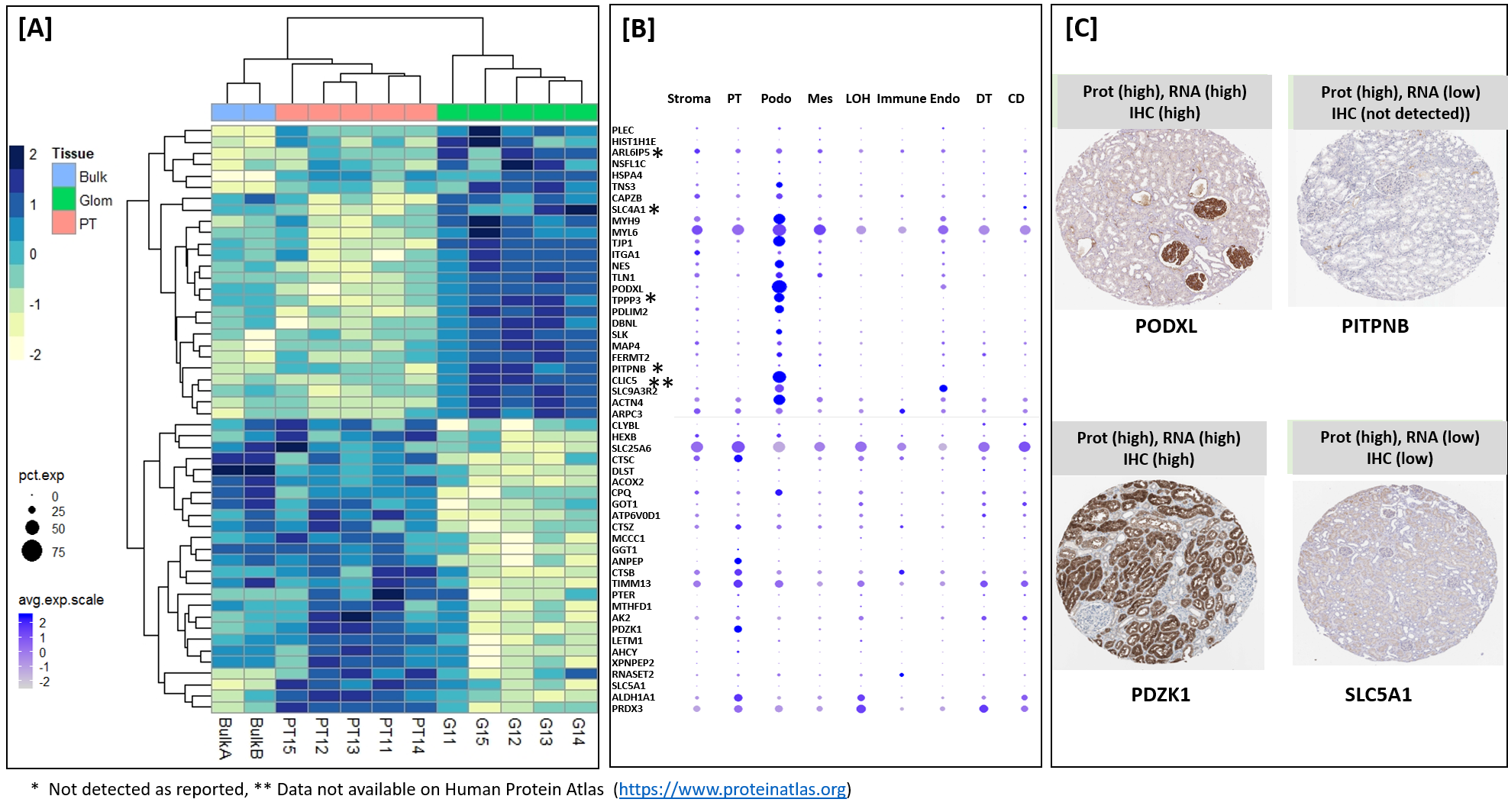
Conclusion: This pilot study provided strategy for analysis of other renal micro-compartments, on a larger scale, to unravel perturbations of renal sub-cellular function in the normal kidney as well as different etiologies of acute and chronic kidney disease and rejection in kidney trannsplantation.
IDDK NIHUG3 -DK114937 and DP3 DK110844.
