Single cell immune profiling of human intestinal allografts reveals heterogeneity and alloreactivity of recipient resident memory T cells in association with graft outcomes
Jianing Fu1, Zicheng Wang2, Mercedes Martinez3, Kristjana Frangaj1, Rebecca Jones1, Xinzheng V Guo4, Aleksandar Obradovic1, Wenji Ma2, Kortney Rogers1, Tomoaki Kato5, Yufeng Shen2, Megan Sykes1,5,6.
1Columbia Center for Translational Immunology, Department of Medicine, Columbia University, New York, NY, United States; 2Department of Systems Biology, Columbia University, New York, NY, United States; 3Department of Pediatrics, Columbia University, New York, NY, United States; 4Human Immune Monitoring Core, Department of Medicine, Columbia University, New York, NY, United States; 5Department of Surgery, Columbia University, New York, NY, United States; 6Department of Microbiology & Immunology, Columbia University, New York, NY, United States
Host-versus-graft (HvG) T cell clones are enriched in the graft mucosa during early rejection after human intestinal transplantation and persist despite rejection resolution. Recipient T cells in the mucosa eventually took on resident memory T cell (TRM) features, likely posing a constant threat of late rejection.
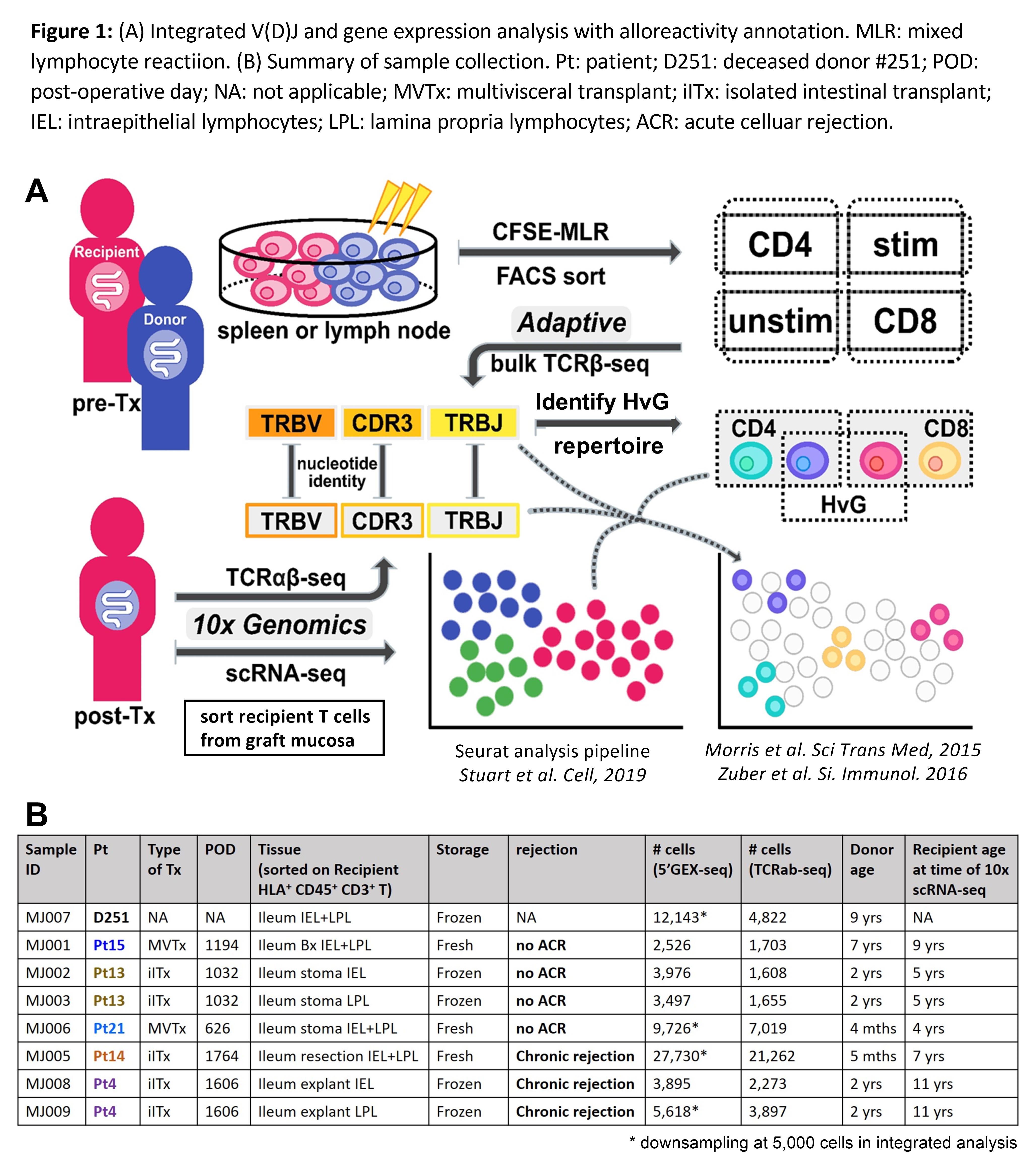
To directly address this hypothesis, we integrated clonotype, alloreactivity and gene expression profiles of FACS sorted allograft recipient T cells at the single cell level to assess the functional phenotype of alloreactive T cells in association with graft outcomes using the 10x Genomics single cell RNA sequencing and our published method1,2 for identifying alloreactive TCR repertoires from pre-transplant mixed lymphocyte reactions (pre-Tx MLRs) (Fig. 1A).
Recipient T cells sorted from graft mucosa of three quiescent and two chronically rejecting allografts and a deceased donor intestine (Fig. 1B) shared at least four clusters (Fig. 2): multifunctional (IL17A+, IL22+, TNF+) TRMs (CD69+, ITGAE+, CXCR6+, PRDM1+) that were enriched for HvG clones (cluster 0); cytotoxic γδ and CD8 TRMs (GZMA+, GNLY+) (cluster 2); nonTRMs (CCR7+, KLF2+, S1PR1+, SELL+) (cluster 4); and regulatory T cells (Foxp3+, CTLA4+) (cluster 13). Cytotoxic (EOMES+, GZMB+, PRF1+) effector T cells (Teff) that were enriched for CD8 HvG clones were identified only in a graft with chronic rejection (Pt14 cluster 1). γδ TRMs that highly express TCF7, CD28H, PRF1 and IL-2 (cluster 11) and CD4 Teff cells enriched for HvG clones expressing RUNX3, RORA, AHR, IL23 and IFNG (cluster 6) were identified only in a graft with chronic rejection (Pt4). T follicular helper (Tfh) cells (BCL6+, CXCR5+, PDCD1+, IL21+) lacking a TRM signature (cluster 3) that were enriched for CD4 HvG clones were identified in a quiescent graft (Pt21) with mucosal lymphoid follicles. Hyporesponsiveness of circulating recipient T cells to donor versus third-party antigens in post-Tx MLR indicated partial tolerance of circulating recipient T cells to donor antigens post-Tx. In line with these observations in circulation, there was a significantly decreased likelihood of detecting HvG clones defined by post- compared to pre-Tx MLR in late quiescent (but not chronically rejecting) allografts (Fig. 3A). Furthermore, quiescent allografts contained a significantly greater percentage of pre-Tx HvG clones that became tolerant in post-Tx MLR compared to chronically rejecting allografts (Fig. 3B).
Our data integrate T cell alloreactivity with clonotype and gene expression profiles at the single cell level. We have demonstrated heterogeneous contributions of pre-existing HvG-reactive T cells to TRM and Tfh compartments in quiescent allografts and evidence for donor-specific tolerance locally and systemically. Chronic rejection is associated with unique CD4 and CD8 Teff cells that are enriched for pre-existing HvG clones and cytotoxic γδ TRMs.


The study is part of a Program Project Grant (PPG) P01 AI106697, funded by the National Institute of Allergy and Infectious Diseases (NIAID). Research reported here was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health (NIH) awards S10RR027050 and S10OD020056..
[1] Morris H. and DeWolf S. et al. Science Translational Medicine. 2015 Jan 28;7(272):272ra10.
[2] Zuber J. et al. Science Immunology. 2016 Oct;1(4). pii: eaah3732.
