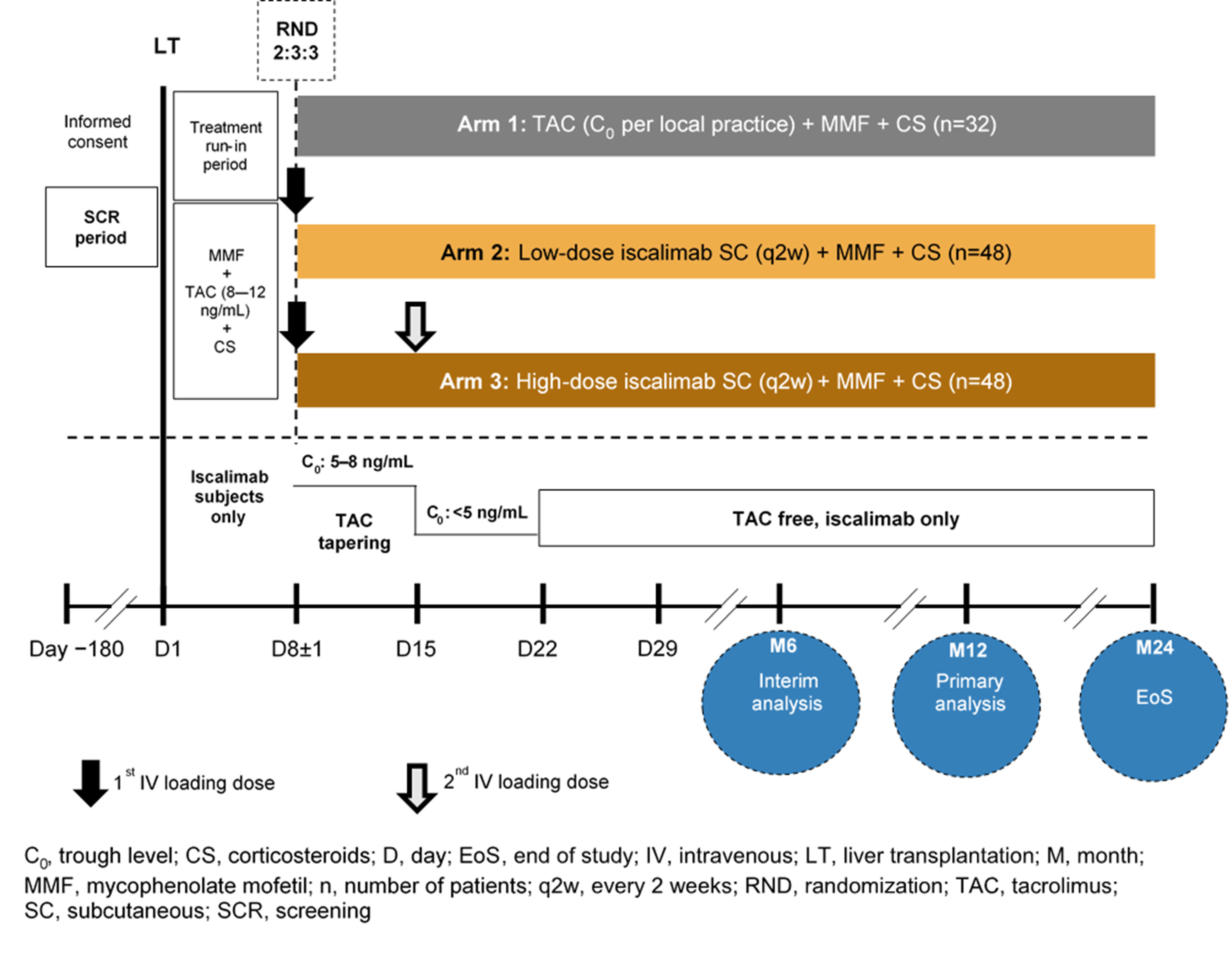
Phase 2 study design evaluating efficacy, safety, pharmacokinetics and pharmacodynamics of the anti-CD40 monoclonal antibody CFZ533 (iscalimab) in de novo liver transplant recipients: The CONTRAIL I study
Björn Nashan1, Stuart J. Knechtle2, Faouzi Saliba3, Ute Laessing4, Pascal Espie4, Jan Klatt4, Sandy Feng5.
1University of Science and Technology of China, Hefei, People's Republic of China; 2School of Medicine, Duke University, Durham, NC, United States; 3Centre Hépato-Biliaire, Hôpital Paul Brousse, Université Paris-Saclay, Villejuif, France; 4Novartis Pharma AG, Basel, Switzerland; 5University of California San Francisco, San Francisco, CA, United States
Introduction: Development of calcineurin inhibitor (CNI)-sparing regimens that can provide long-term renal and other benefits while preserving anti-rejection efficacy remains a key unmet medical need in liver transplantation (LT). CFZ533 (iscalimab), a fully human anti-CD40 monoclonal antibody, has been shown to prolong allograft survival in preclinical models and block T cell-dependent antibody responses in humans. Here, the rationale and design of the CONTRAIL I study are presented. This Phase 2 study aims to evaluate the efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) of high and low doses of iscalimab versus standard tacrolimus (TAC) regimen in de novo liver transplant recipients (LTRs).
Materials and Methods: CONTRAIL I (NCT03781414) is a 12-month, multicenter, open-label study with a 12 month follow-up period. De novo LTRs aged 18–70 years receiving grafts from deceased donors with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 will be randomized (2:3:3) on Day 8±1 post-LT to standard TAC, low-dose iscalimab, or high-dose iscalimab (Figure). All patients will receive mycophenolate mofetil throughout the study and steroids for the first 4 months following LT. The primary objective is to evaluate the rate of composite efficacy failure (biopsy-proven acute rejection (rejection activity index ≥3], graft loss, or death) at 12 months. Key secondary objectives will include efficacy event rates, renal function (evolution of eGFR), safety and tolerability, PK, and PD (soluble CD40 in plasma) of iscalimab over 12 and 24 months. Key exploratory objective is to evaluate differences in liver histopathology with high and low doses of iscalimab versus a standard TAC regimen (using Banff, rejection activity index, modified hepatic activity index, and liver fibrosis score) at 12 months post-transplantation.
Results and Discussion: At least 128 LTRs will be randomized from multiple centers in Europe and North America. The first patient first visit was achieved on October 2019; currently the study is recruiting patients, and study completion is expected by Q4 2022.
Conclusion: The study findings will identify the iscalimab dose for further clinical development and inform the safety and efficacy of iscalimab as a CNI-free regimen in de novo LTRs.