Impact of enhanced recovery after surgery (ERAS) protocols on robotic living kidney donor and recipient outcomes
Brianna Ruch1, Amit Sharma1, Adrian Cotterell1, Jeanette Amery1, Aamir Khan1, Seung D. Lee1, Michael Scott1, Chandra Bhati1, Marlon Levy1.
1Hume-Lee Transplant Center, Virginia Commonwealth University, Richmond, VA, United States
Introduction: Traditional management during living donor nephrectomies includes intraoperative volume loading to maintain renal perfusion and urine output. Studies have shown that fluid restriction in donor-recipient pairs leads to increase in delayed graft function after laparoscopic nephrectomy (1). In 2019, our center initiated intraoperative fluid minimisation as part of an enhanced recovery after surgery (ERAS) protocol for all robotic living donor nephrectomies (RLDN). We hypothesized that fluid restriction in robotic kidney donors may affect their renal function and this in turn could negatively impact the outcomes of the transplanted renal allograft.
Methods: A retrospective analysis of all RLDN and corresponding renal transplant recipients performed at our single center from 01/2016 to 10/2019 was done. Pre-ERAS (2016-2018) and post-ERAS outcomes (2019) were compared using standard t-test for unequal variances. Robotically implanted recipients and their corresponding donors were excluded from this analysis. A p-value < 0.05 was considered significant.
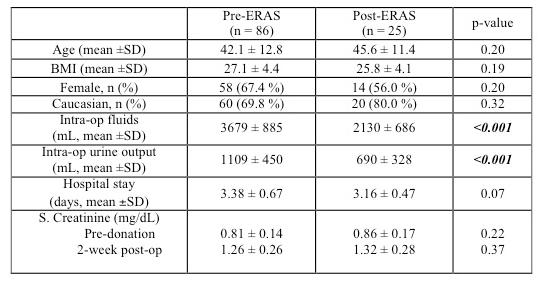
Results and discussion: A total of 111 robotic live donor nephrectomies and corresponding renal transplants were performed (Pre-ERAS - 86; Post-ERAS - 25). The groups were comparable in terms of age, gender, BMI and race. Post-ERAS, there was a trend towards a decrease in donor hospital stay (3.16 ± 0.57 days vs. 3.38 ± 0.67 days, p-value 0.06). There was a significant decrease in intraoperative fluid administration (2130 ± 686 mL vs 3679 ± 885 mL, p-value < 0.001) and urine output (690 ± 328 mL vs 1109 ± 450 mL, p-value < 0.001) in the post-ERAS group compared to pre-ERAS. However, this did not have any negative effect on donor renal function. The 2-week serum creatinine was not statistically different pre-ERAS and post-ERAS (1.26 ± 0.26 vs. 1.32 ± 0.28 mg/dL, p-value 0.37).
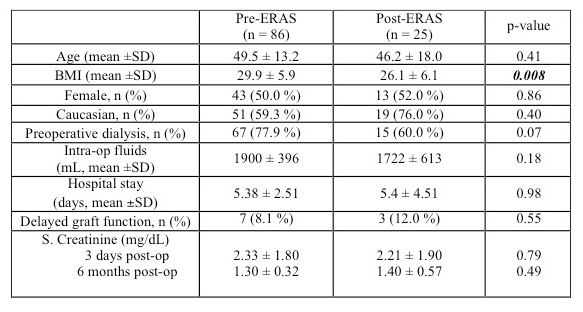
Preoperative demographics for the transplant recipients in the pre- and post-ERAS groups were comparable for age, gender and race. Post-ERAS recipients had lower BMI (26.1 ± 6.1 vs. 29.9 ± 5.9, p 0.008). Both groups of recipients received comparable fluids volumes intraoperatively. The recipient renal function was not impacted by fluid restriction in the robotic donors as reflected in the rates of DGF of 8% vs. 12% in pre- and post ERAS groups respectively (p-value 0.55). The 6-month serum creatinine pre- and post-ERAS was not significantly different (1.30 ± 0.32 vs. 1.40 ± 0.57, p-value 0.49).
Conclusions: Intraoperative fluid minimisation during robotic living donor nephrectomy did not have a deleterious effect on the post-operative renal function of either the robotic donors or their corresponding renal transplant recipients. Implementation of enhanced recovery protocols for robotic living donor nephrectomies did show a trend towards decreased hospital stay of the kidney donors. We recommend that larger multi-center studies should be performed to confirm our findings.
[1] Nieuwenhuijs-Moeke GJ, Huijink TM, Pol RA, et al. J Clin Med. 2019;8(10):1587.
