Successful induction of hematopoietic chimerism by MCL-1 and Bcl-2 inhibition without radiation/chemo therapy in nonhuman primates
David Ma1, Takayuki Hirose1, Tatsuo Kawai1.
1Center for Transplant Sciences, Massachusetts General Hospital, Boston, MA, United States
Allograft tolerance in clinical HLA mismatched kidney transplantation has been achieved by induction of hematopoietic chimerism via donor bone marrow transplantation (BMT). However, wider clinical application of tolerance regimens has been hampered by the myelosuppressive toxicity of the radiation or chemotherapy used in conditioning. We have previously described methods of successfully reducing the requisite doses of total body irradiation (TBI) by using Venetoclax (ABT-199), a small molecule inhibitor of the anti-apoptotic protein BCL-2. However, a minimal dose of TBI 1.5Gy was still required for chimerism induction. Since Mcl-1, another member of the BCL-2 family proteins, is highly expressed in hematopoietic stem cells (HSC), we hypothesized that Mcl-1 inhibition might delete the host HSC niche, thus facilitating donor bone marrow engraftment without TBI requirement.
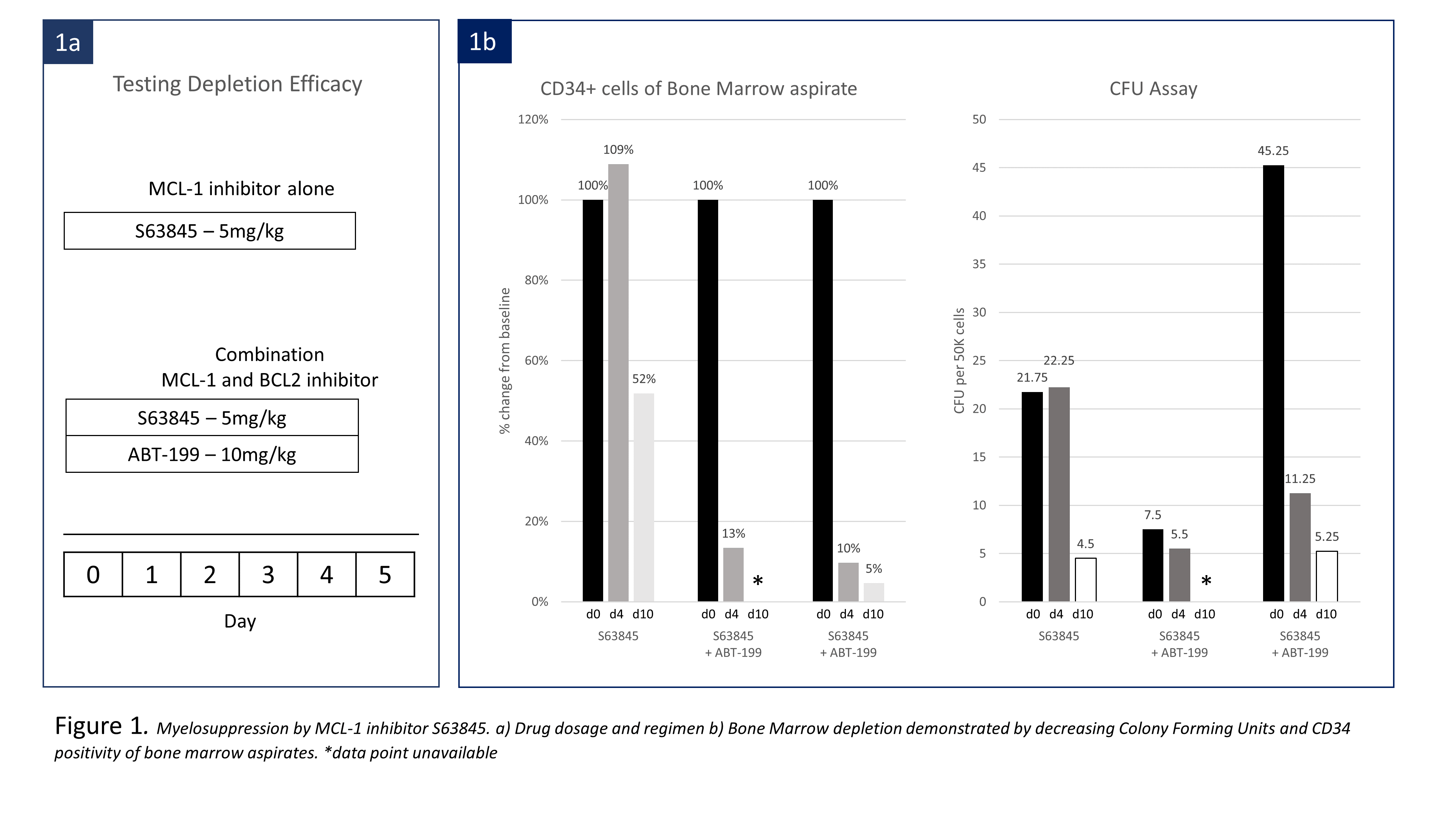
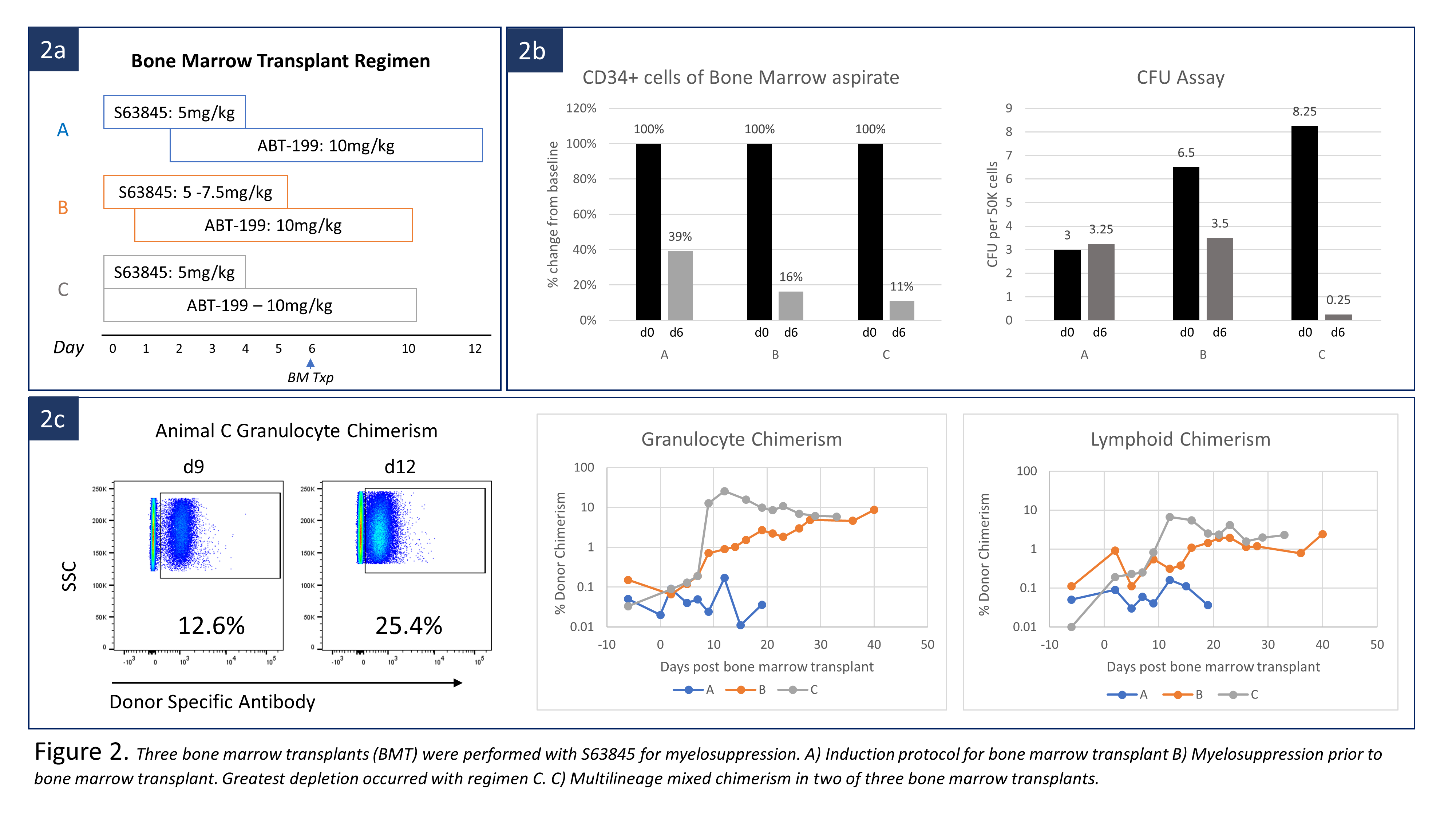
We first tested HSC depleting potential by Mcl-1 inhibition without BMT. Three cynomolgus macaques were treated with either Mcl-1 inhibitor (S63845) alone or combined with ABT-199 (Fig. 1a). BMT from an MHC mismatched donor was then performed after conditioning with S63945 and ABT-199 in three monkeys. All recipients were treated with pre-BMT ATG and post-BMT anti-CD154 and cyclosporine, all of which were required for chimerism induction in our previous studies. Three different timing and dosing regimens of S63845 and ABT-199 were used with BMT (Fig 2a).
In depletion efficacy testing, administration of S63845 alone depleted HSCs, measured as CD34 positivity in bone marrow aspirates, to 51% of pretreatment levels. Total colony forming units (CFU) was suppressed to 45%. Combining ABT-199 with S63845 decreased HSCs in two animals to 22% and 2% by day 6 (Fig. 2b). When performing BMT, delayed ABT-199 administration with S63845 (Regimen A), depleted HSC to 40% and no hematopoietic chimerism was induced. S63845 dosage was increased to 7.5mg/kg on days 0 and 1(Regimen B). At the time of BMT, CFU counts reduced by 50%, and HSC were depleted to 16% of baseline. Multilineage chimerism appeared on day 9. Maximal granulocyte and lymphoid chimerism was 8.62% and 2.42% respectively by day 40 (Fig. 2c). Simultaneous administration of S63845 and ABT-199 (Regimen C) deleted HSCs to 11% and suppressed CFU counts to 3% of baseline. Multilineage chimerism appeared on day 7, with granulocyte and lymphoid chimerism as high as 25% and 6.6% on day 12 (Fig.2c). Both recipients remain chimeric at day 33 and 40. Neither S63845 nor the S63845/ABT-199 combination showed significant toxicity beyond transient transaminitis and elevated amylase.
Combined inhibition of Mcl-1 and Bcl-2 resulted in HSC deletion, making hematopoietic chimerism induction possible without chemo/radiation therapy. Although optimal dose of S63848 remains to be defined, this approach may be widely applicable not only to transplant tolerance induction but to allogeneic or autologous BMT for hematologic diseases or gene transfer.
There are no comments yet...
