Sinomenine enhances imDC derived from IPS cell-directed differentiation to induce transplant immune hyporesponsiveness in mouse
Cuixiang Xu1,2, Zhankui Jin1, Puxun Tian2, Feng Han1.
1Center of Shaanxi Provincial Clinical Laboratory, Shaanxi Provincial People’s Hospital, Xi'an, People's Republic of China; 2Kidney Transplantation, First Affiliated Hospital of Medical College of Xi’an Jiaotong University, Xi’an , People's Republic of China
Introduction: Induced pluripotent stem cells (iPS) have a wide source and is not restricted by ethics, so that it is expected to become a new source of immature dendritic cells (imDCs). The Chinese medicine monomer sinomenine (SN) can reduce the expression of co-stimulatory molecules on the surface of imDCs, thereby inhibiting the maturation of imDCs. Therefore, in this study, we intend to explore the process of SN acting on induced pluripotent stem (iPS) to differentiate into imDCs, and in order to obtain imDCs (SN-iPS-imDCs) that can remain immature for a long time.
Methods: IPS cells were differentiated into imDCs (SN-iPS-imDCs and iPS-imDCs) with three-steps differentiation method with SN. A mouse skin transplantation model was established; SN-iPS-imDCs (1×106 cells) were infused into the mouse skin transplantation model, and 5 groups of controls were established; the skin rejection score, graft survival rate and body weight were observed in recipient mouse; the serum levels of cytokines, the proportion of lymphocyte subsets CD4+CD25+Foxp3+Tregs in the spleen, and the relative expression of Foxp3 mRNA were detected in the recipient mouse; unrelated third-party lymphocytes were added to the lymphocyte mixed culture, and the specificity of induced transplant immunoreactivity by SN-iPS-imDCs and iPS-imDCs were observed; the tissue destruction and inflammatory cell infiltration in the skin graft were detected by histopathology.
Results: A mouse skin transplantation model was successfully established. The scores of skin rejection in the SN-iPS-imDCs group were significantly lower than those in the PBS, SN and iPS-imDCs groups at each time point after surgery; on day 28 of transplantation, the body weight of the SN-iPS-imDCs group was increased significantly compared with day 0 (P<0.05); survival analysis showed that the survival time of skin grafts in the SN-iPS-imDCs group was significantly longer than that in the SN, iPS-imDCs and PBS groups (P<0.05);
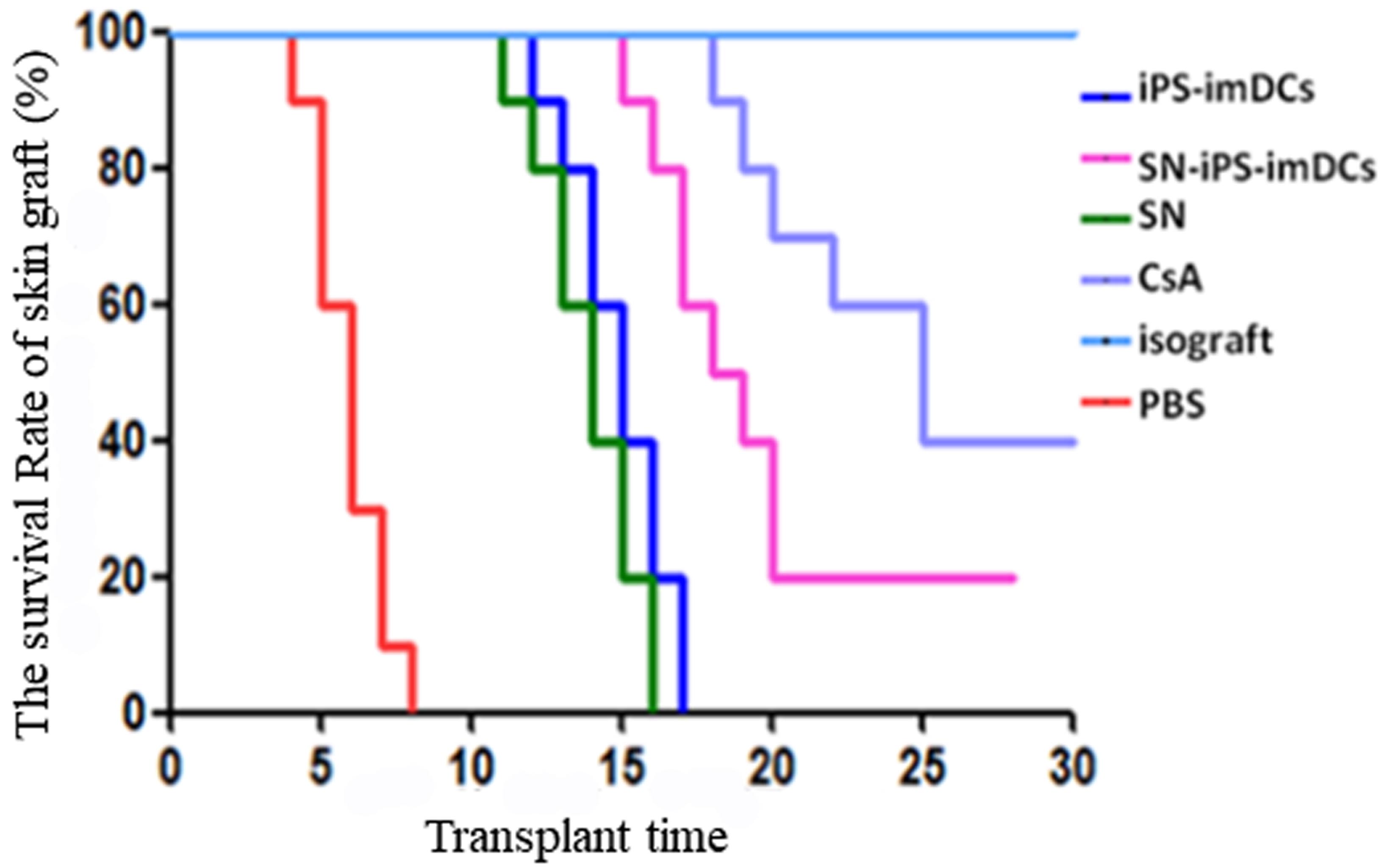
from the 3rd day to the 7th day of the transplantation, the expression levels of IL-12, IL-2 and IFN-γ in the serum of each group were increased except the CsA group, and the elevation level in the SN-iPS-imDCs group was significantly lower than that in the SN, iPS-imDCs and PBS groups (P<0.05); the serum IL-10 levels in the SN-iPS-imDCs group were second only to the CsA group but significantly higher than those in the PBS, iPS-imDCs and SN groups (P<0.05); in the SN-iPS-imDCs group, the spleen CD4+CD25+Tregs and CD4+CD25+Foxp3+Tregs accounted for the highest proportion of CD4+T cells, and there were significant differences compared with other groups (P<0.05); the Foxp3 mRNA expression in SN-iPS-imDCs group was significantly higher than that in iPS-imDCs group (P <0.05); on day 7 of transplantation, spleen T cells in SN-iPS-imDCs group and iPS-imDCs group were significantly less reactive to donor lymphocytes than unrelated third-party C3H mouse. The SN-iPS-imDCs group had lower response to donor lymphocytes than the iPS-imDC group (P<0.05). HE staining of skin grafts showed that the inflammatory response of the grafts in the SN-iPS-imDCs group was significantly lower than that in the iPS-imDCs and PBS groups.
Conclusions: Infusion of donor-derived SN-iPS-imDCs could induce donor-specific transplantation immunoreactivity, could reduce the incidence of skin graft rejection, and could prolong the survival time of transplanted skin grafts.
National Nature Science Foundation of China (No. 81900686). National Nature Science Foundation of China (No. 81100179).
There are no comments yet...
