
Prolonged survival of genetically modified pig livers during machine perfusion with human blood
Taylor M. Coe1, Danielle Detelich1, Charles G. Rickert1, Cailah Carroll1, Nikolaos Serifis1, Rudy Matheson1, Siavash Raigani1, Ivy Rosales1, Wenning Qin2, Yinan Kan2, Jacob Layer2, Michele Youd2, William Westlin2, Shoko Kimura1, Agnes Azimzadeh1, Luhan Yang2, James F. Markmann1.
1Surgery, Massachusetts General Hospital, Boston, MA, United States; 2eGenesis, Cambridge, MA, United States
Introduction: Pig-to-human liver xenotransplantation provides a potential solution to the organ shortage problem. Barriers remain, including profound thrombocytopenia, coagulopathy, and rejection. We utilized ex-vivo porcine liver perfusion with human blood to study the human response to genetically modified pig livers.
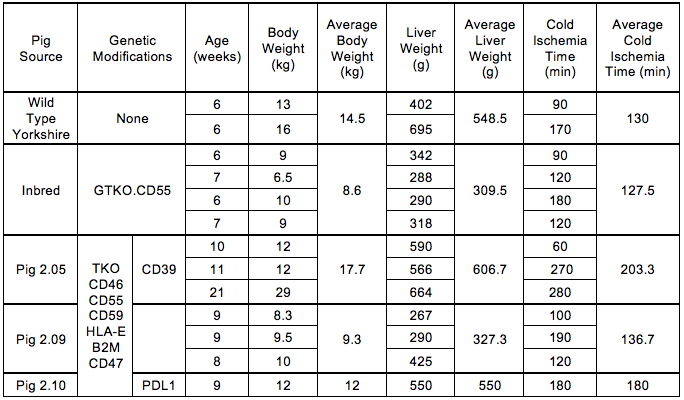
Materials and Methods: Livers from wild type pigs (WT) (n=2), GTKO.CD55 pigs (n=4) and three variations of multiplex genome engineered pigs (Pig 2.0; n=7) that contained triple knockout for aGal, b4Gal, CMAH plus different genetic modifications targeting complement, immune and inflammation regulation, underwent normothermic machine perfusion using freshly collected, heparinized human whole blood and plasma on the Liver Assist (Organ Assist, Groningen, NL). Perfusion was continued until graft failure, defined by decreased blood flow secondary to elevated vascular resistance. Laboratory analyses including lactate, AST, ALT, CBC were performed. Tissue underwent H&E, IgG, IgM and C4d staining.
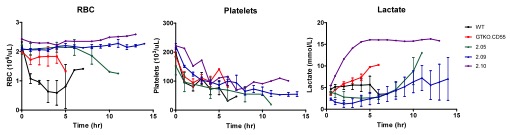
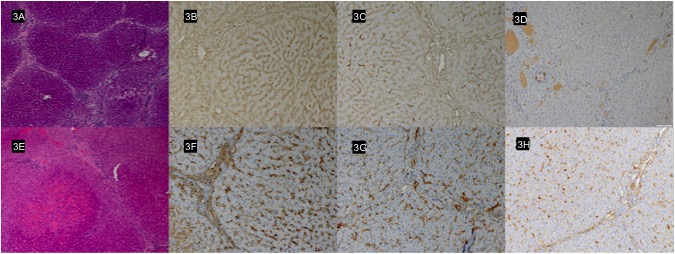
Results: Liver characteristics are shown in Table 1. All livers demonstrated progressively decreasing blood flow. However, this was evident earlier and progressed faster in the WT and GTKO.CD55 groups compared to the Pig 2.0 group, which correlated to longer survival of the Pig 2.0 livers (Fig 1). Mean graft survival was 6 hours in WT livers, 4.5 hours in GTKO.CD55 livers, 9.7 hours in pig 2.05, 13 hours in pig 2.09 and 13 hours in pig 2.10. The total red blood cell count remained more stable over time in the genetically modified livers while the platelet count decreased in all livers. The lactate decreased and remained lower in the 2.05 and 2.09 livers when compared to the WT and GTKO.CD55 livers (Fig 2). Histologically, Pig 2.0 livers exhibited preserved hepatic architecture with mild diffuse portal and sinusoidal inflammation (Fig 3A). Diffuse mild sinusoidal IgG and IgM staining confirmed antibody deposition (Fig 3B and 3C), but C4d was negative (Fig 3D). In contrast, WT livers sustained focal ischemic necrosis and vascular congestion (Fig 3E), with a greater intensity of IgG and IgM staining (Fig 3F and 3G) and C4d positivity (Fig 3H).
Discussion: In this ex vivo perfusion model, Pig 2.0 livers with genetic modifications targeting complement, immune and inflammation regulation achieved prolonged function, improved lactate clearance, a more stable red blood cell count and diminished antibody and complement deposition compared to WT or GTKO.hCD55 xenografts. The prompt and profound platelet loss was not attenuated with the current modifications that do not address coagulation dysregulation.
Conclusion: This normothermic machine perfusion model is an efficient and informative tool to simulate pig-to-human xenotransplantation and evaluate the efficacy of different genetic constructs, providing insight to the use of genetically modified porcine livers as an ex vivo perfusion bridge to liver transplantation.

[1] Shah JA, Patel MS, Elias N, et al. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant 2017; 17(8):2178-2185.
[2] Marecki H, Bozorgzadeh A, Porte RJ, et al. Liver ex situ machine perfusion preservation: A review of the methodology and results of large animal studies and clinical trials. Liver Transept 2017; 23(5):679-695.