Prolonged survival and attenuated pulmonary vascular resistance rise in a multitransgenic pig ex vivo lung xenoperfusion model
Margaret Connolly1, Lars Burdorf1, Aspen Pierson1, Madelyn Ma1, Kaitlyn Petitpas1, Zahra Abady1, Shannon Pratts1, Wenning Qin2, Yinan Kan2, Jacob Layer2, Michele Youd2, William Westlin2, Luhan Yang2, Agnes Azimzadeh1, Richard N. Pierson III1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2eGenesis Inc., eGenesis, Boston, MA, United States
Introduction: Ex vivo perfusion of porcine lungs with human blood reliably evaluates mechanisms of xenograft failure. New advances have led to the development of Pig 2.0 with multiple xeno-targeted genetic modfifications. Physiologic behavior of Pig 2.0 lungs has not previously been evaluated.
Materials and Methods: Lungs from Pig 2.0 with combined Gal1,3αGal, β4Gal, and Neu5Gc triple-knockouts (TKO), addition of human complement pathway regulatory proteins (hCPRPs: hCD46, hCD55, hCD59), and transgenes targeting innate immunity (CD47, HLA-E/b2M) were perfused with human blood for up to 8 hours using an ex vivo perfusion circuit. Pig 2.09 had high hCPRP expression as well as moderate CD47, HLA-E/b2M, while Pig 2.10 had moderate hCPRP expression with high expression of CD47, HLA-E/b2M, and hPDL1. GTKO.hCD55 lungs served as a reference group. In each pair of lungs, blood was left ‘untreated’ on one side, and the other side was ‘treated’ with 1-BIA, a thromboxane synthase inhibitor, and histamine (H) receptor blockade.
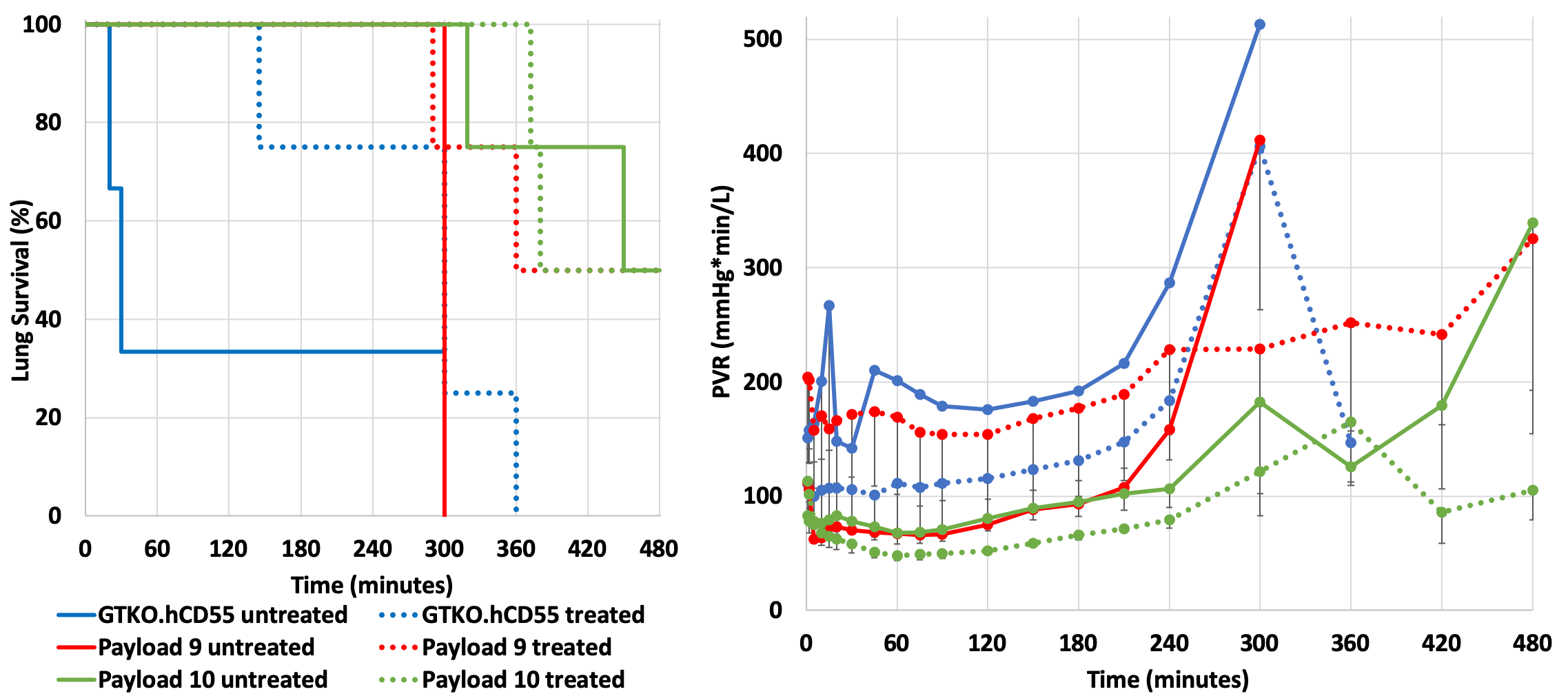
Results: Median survival time for untreated lungs was significantly higher for Pig 2.10 lungs (465 min, range 319-480 min, n=4) compared to reference lungs (30 min, range 20-300 min, n=3) (p=0.02). When lungs were treated with 1-BIA and H-blockade, median survival was similar for Pig 2.09 (420 min, range 290-480 min, n=4) and Pig 2.10 (430 min, range 372-480, n=4) compared to the reference (300 min, range 145-360 min, n=4) (p=0.06) (Fig.1A). Pulmonary vascular resistance (PVR) rise was significantly attenuated and delayed in untreated Pig 2.10 lungs relative to GTKO.hCD55 lungs (p<0.001), which was replicated when blood was treated with 1-BIA/H-blockade (p=0.002) (Fig.1B).
Platelet and granulocyte sequestration occurred within 5-15 min of reference lung perfusion and were not attenuated in association with Pig 2.0 lungs. The rise in C3a was low to moderate in all groups, however, some early lung failures were associated with higher C3a levels.
Discussion: TKO combined with human complement- and immune-related regulatory transgenes protect lungs from PVR increase and improve lung survival during human blood perfusion similar to anti-inflammatory drug treatment in reference lungs. Pig 2.10 (with moderate hCPRP expression and higher expression of innate immunity: CD47, HLA-E/b2M) demonstrated low PVR over a longer period than Pig 2.09 (with high hCPRP expression and lower expression of innate immunity targets). Platelet and granulocyte sequestration were not prevented, as previously described with other lung genetics.
Conclusion: Pig 2.0 lungs with higher expression of innate immunity genetic targets appear to be protected from early xenograft injury compared to reference lungs. The transgene combination expressed by Pig 2.0 may be helpful to achieve successful xenotransplantation of the lung and other organs, but additional regulation of coagulation and inhibition of adhesion pathways may be required for further improvement.
