Analyzing interactions between donor endothelium and recipient blood under flow conditions in vitro
Margaret Connolly1, Kaitlyn Petitpas1, Lars Burdorf1, Zahra Abady1, Madelyn Ma1, Benjamin Cerel2, Christopher Laird2, Donald Harris2, Agnes Azimzadeh1, Richard N. Pierson III1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2Department of Surgery, University of Maryland, Baltimore, MD, United States
Introduction: The Bioflux system has been used to study platelet-driven thrombosis and leukocyte adhesion. Typically, whole blood is stained with calcein (green) to evaluate platelet adhesion and aggregation but this technique does not distinguish adherent leukocytes from larger platelet aggregates. We sought to develop a method to separately evaluate leukocyte (WBC) adhesion, which also take up calcein when whole blood is stained, while simultaneously measuring platelet aggregation during interactions between porcine endothelium and whole human blood.
Materials and Methods: Porcine endothelia were cultured to confluence in microfluidic channels and then perfused with fluorescently stained human blood. Blood was separated into two conditions. In one condition, whole blood was stained with calcein for 30 minutes (standard method). In the revised method, whole blood was stained with both calcein and with Vybrant DiD for 30 minutes. The blood was then perfused at 5 dynes/cm2 and 37oC for one hour to mimic microcirculatory flow. Video images generated with the Montage software (Fluxion Biosciences) were overlaid to provide a visual representation of the staining.
Results: Whole blood stained with calcein demonstrates heterogeneous green staining with a fine-grained speckled pattern (presumably reflecting individual adherent platelets) and larger particles reflecting adherent WBCs or platelet aggregates (Fig. 1A). When WBCs are also labeled with DiD (red), the calcein-labeled platelet aggregates are readily distinguished from the adherent WBCs, and in some cases WBCs can be seen within larger platelet thrombi (Fig. 1B).
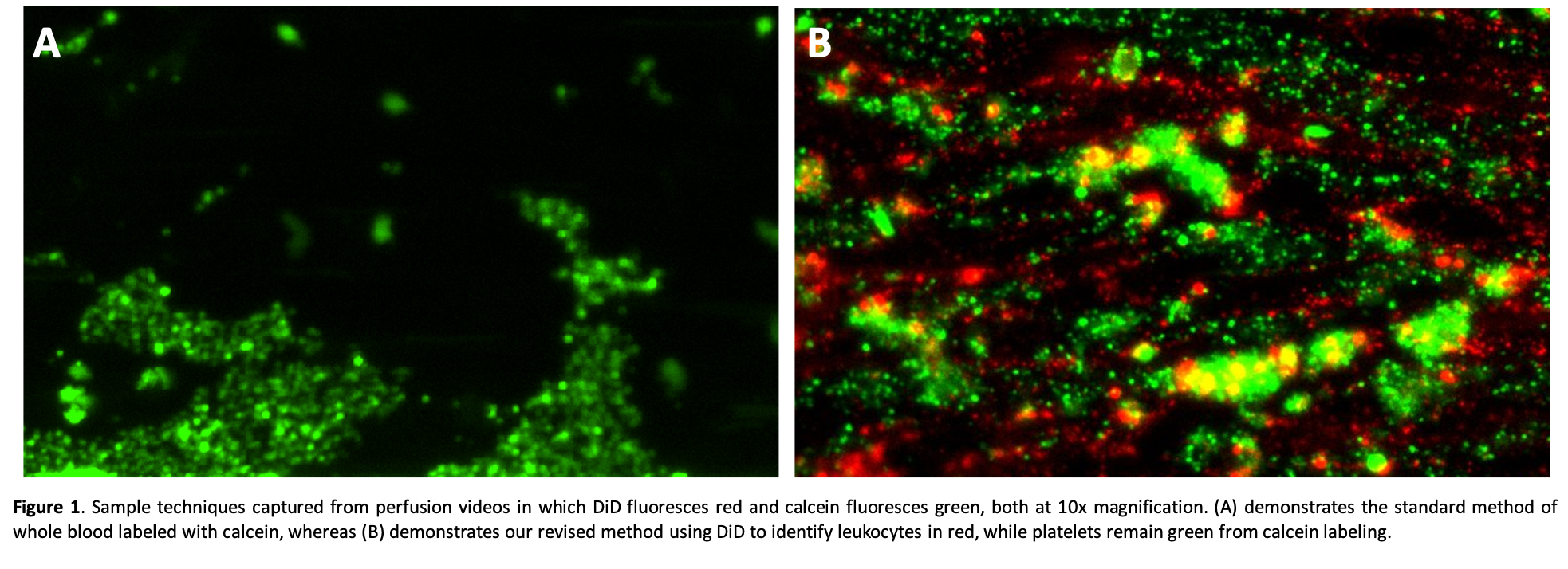
These separate cell staining techniques are supported by preliminary flow cytometry analyses and have been replicated in conditions in which blood components are separately stained and then recombined to form whole blood.
Discussion: The combined use of calcein and Vybrant DiD to stain whole blood to separately identify platelets and leukocytes allows both visual and quantitative measurements of the activity of each cell population during one in vitro perfusion experiment. This adds information useful to study the cell adhesion mechanisms associated with cross-species coagulation pathway dysregulation (physiologically inappropriate thrombosis, platelet activation and adhesion) and integrin- and selectin-mediated WBC activation and adhesive interactions.
Conclusion: Simultaneous use of different fluorescent stains can be applied to efficiently evaluate potentially important interactions between blood cell populations that may contribute to graft injury mechanisms using a small sample of donor endothelial cells and a few milliliters of recipient blood.
There are no comments yet...
