
Liver transplantation of hepatitis C-viremic donors to hepatitis C naive recipients
Fidel Lopez-Verdugo1, Shannon Sell1, Jake Krong1, Gordon Harmston1, Andrew Gagnon1, Mark E. Boschert1, Ivan Zendejas1, Edward J. Frech1, Shiro Fujita1, Robert Jones1, Manuel I. Rodriguez-Davalos1, Richard K. Gilroy1, Diane Alonso1.
1Transplant Services, Intermountain Medical Center, Salt Lake City, UT, United States
Introduction: The opioid epidemic generates a high number of hepatitis C virus – nucleic antigen testing (HCV-NAT) positive donors. The availability of effective direct-acting antiviral (DAA) therapies, presents an opportunity to expand the donor pool. This study describes outcomes at one of the first centers to begin HCV-NAT positive in NAT negative recipients liver transplantation (LT).
Materials and Methods: Prospective, single center study. HCV-NAT negative and DAA naïve recipients who received a HCV-NAT positive liver were included. For comparative analysis, the cohort was divided in two groups. Patients were classified according to whether they received a HCV-NAT positive liver during the first half (initial experience) or the second half (current experience) of the study period.
Results and Discussion: 22 LT were included. 14 (63.6%) recipients were male, with a median age of 49 years. The most common diagnosis was non-alcoholic steatohepatitis (7/22). Median allocation MELD was 24 (IQR: 21.7-28.2). All donors had positive HCV-NAT. 18 (81.8%) donors were male, with a median age of 32.5 years and median BMI of 25.6 kg/m2. 20 donors (90.9%) had a history of IV drug use and the most common cause of death was drug overdose in 15 (68.2%). Most procurements (59%) were national offers, suggesting that HCV-NAT positive livers are still being discarded at high rates. Recipients underwent NAT testing on POD 4 and all were viremic.
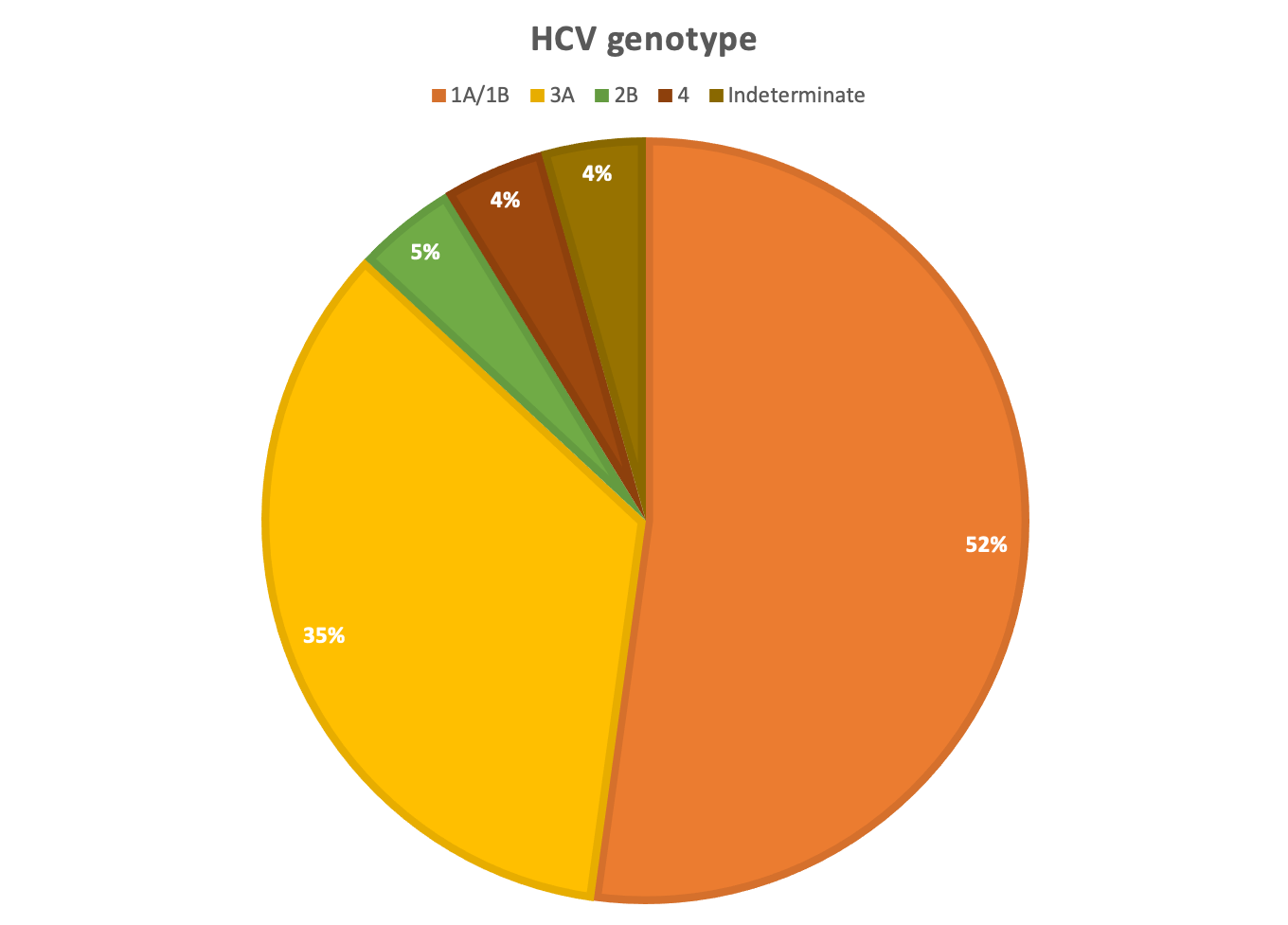
21 patients started DAA therapy at a median time of 58 days (IQR: 32-87) after LT.
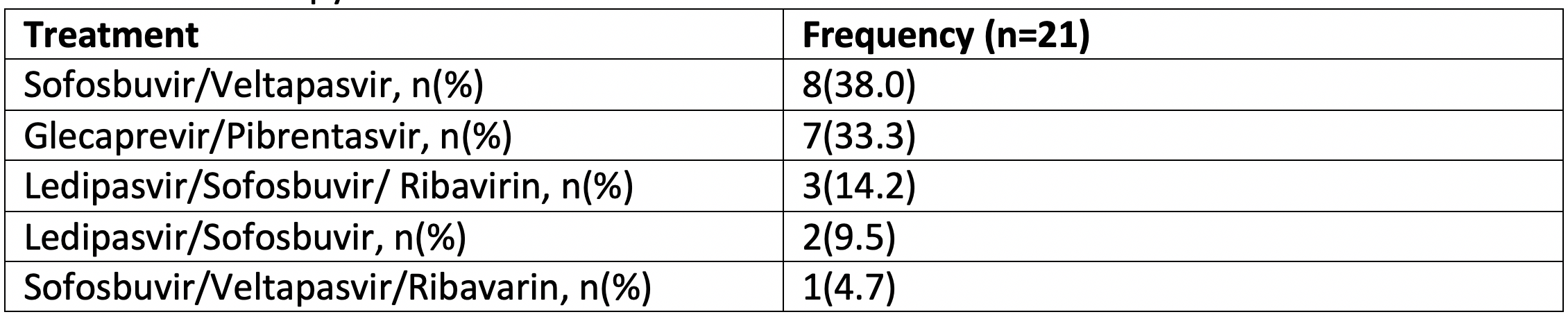
Patients in the current group had a shorter median time to initiation of DAA therapy (32 days; IQR: 17-47.2) when compared to the initial experience group (86 days; IQR: 58-96 [p<0.001]). This was shown to be due to shorter times to DAA application after LT (median 14.5 days; IQR: 8.7-21 vs median 37 days; IQR: 17-43 [p=0.029]) as well as a decrease in time from application submission to insurance approval (median 5 days; IQR: 2-10.5 vs median 26 days; IQR: 14-48.5 [p=0.002]). All patients from the current group received pangenotypic DAA therapy, compared to six (54.5%) from the initial experience (p=0.035). All patients have achieved SVR-12. Overall patient- and graft-survival was 95.4% at a median follow up of 576 days. During the study period we observed a decreasing trend in the median institutional MELD at the time of transplant (p<0.001).
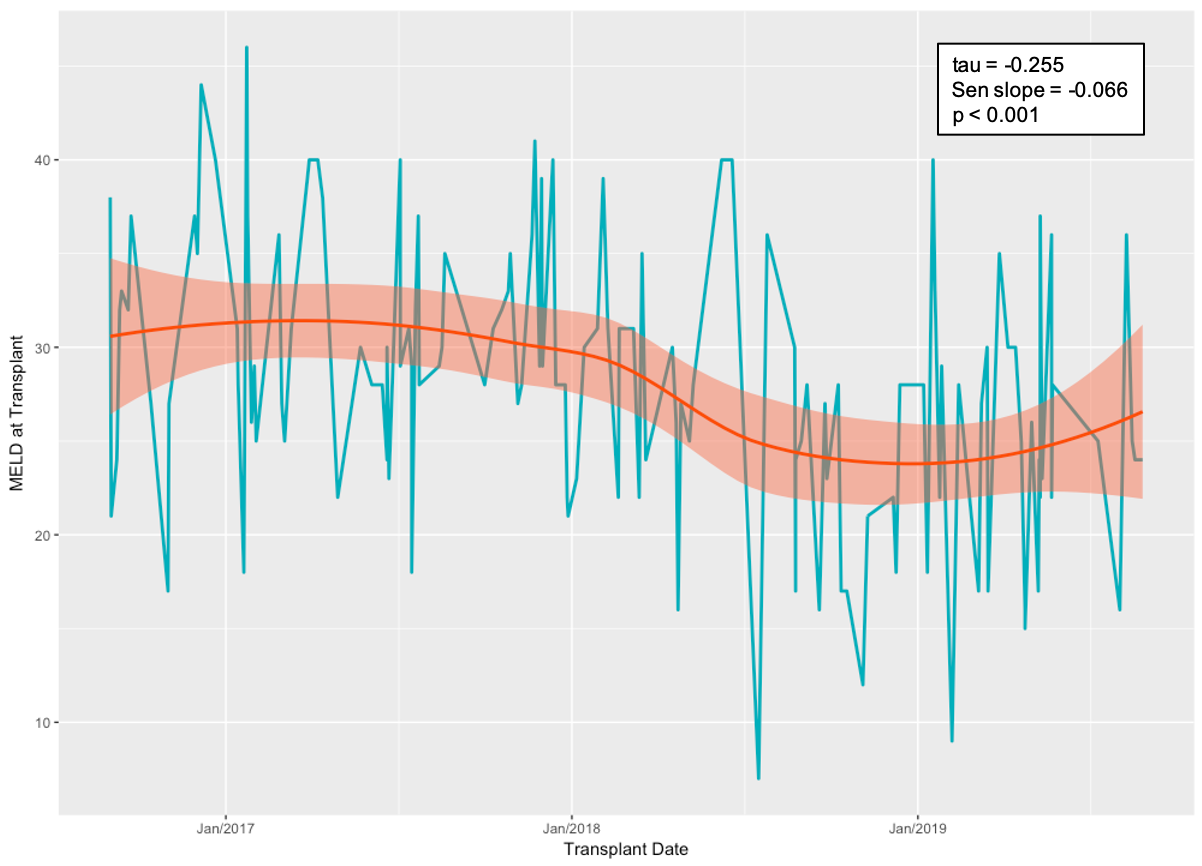
Conclusion: This series shows favorable short- and intermediate-term graft- and patient-survival consistent with national overall LT data. Increased collaboration with insurance companies resulted in shorter times for initiation of DAA therapy. Different strategies have been developed to address the opioid epidemic. As the effect of these policies start to take place, the growth rate of HCV-NAT positive, otherwise healthy donors will become less over time and start to decrease at some point. As such, timely adoption of this practice is crucial.
[1] Sapiano MRP, Jones JM, Bowman J, Levi ME, Basavaraju SV. Impact of US Public Health Service increased risk deceased donor designation on organ utilization. Am J Transplant. 2019 Apr 8. doi: 10.1111/ajt.15388
[2] Gonzalez SA, Trotter JF. The rise of the opioid epidemic and hepatitis C-positive organs: A new era in liver transplantation. Hepatology. 2018;67(4):1600–1608. doi:10.1002/hep.29572
[3] Vukotic R, Conti F, Fagiuoli S, et al. Long-term outcomes of direct acting antivirals in post-transplant advanced hepatitis C virus recurrence and fibrosing cholestatic hepatitis. J Viral Hepat. 2017 Oct;24(10):858-864. doi: 10.1111/jvh.12712
There are no comments yet...